Atropine sulfateCAS# 55-48-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

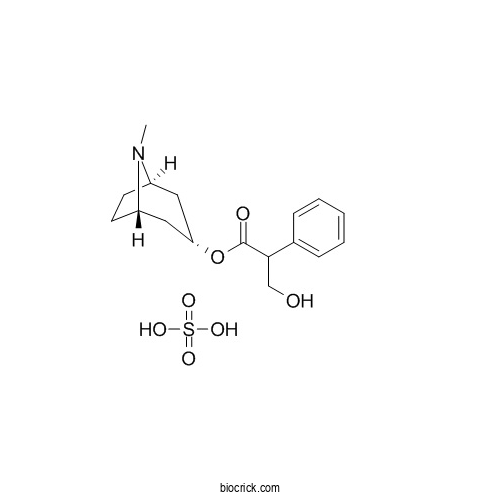
| Cas No. | 55-48-1 | SDF | Download SDF |
| PubChem ID | 17184 | Appearance | White crystalline |
| Formula | C17H25NO7S | M.Wt | 387.45 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Sulfatropinol;5908-99-6 | ||
| Solubility | Freely soluble in water; soluble in ethanol and methan | ||
| Chemical Name | (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 3-hydroxy-2-phenylpropanoate;sulfuric acid | ||
| SMILES | CN1C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3.OS(=O)(=O)O | ||
| Standard InChIKey | VJFQPODMEGSXHC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H23NO3.H2O4S/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12;1-5(2,3)4/h2-6,13-16,19H,7-11H2,1H3;(H2,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Atropine sulfate causes a significant increase in IOP when given both topically and by intramuscular injection. |

Atropine sulfate Dilution Calculator

Atropine sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.581 mL | 12.9049 mL | 25.8098 mL | 51.6196 mL | 64.5245 mL |
| 5 mM | 0.5162 mL | 2.581 mL | 5.162 mL | 10.3239 mL | 12.9049 mL |
| 10 mM | 0.2581 mL | 1.2905 mL | 2.581 mL | 5.162 mL | 6.4524 mL |
| 50 mM | 0.0516 mL | 0.2581 mL | 0.5162 mL | 1.0324 mL | 1.2905 mL |
| 100 mM | 0.0258 mL | 0.129 mL | 0.2581 mL | 0.5162 mL | 0.6452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Atropine sulfate is a competitive muscarinic acetylcholine receptor antagonist. IC50 value: Target: mAChR Atropine is a naturally occurring tropane alkaloid extracted from deadly nightshade (Atropa belladonna), Jimson weed (Datura stramonium), mandrake (Mandragora officinarum) and other plants of the family Solanaceae. Atropine is a competitive antagonist of the muscarinic acetylcholine receptors (acetylcholine being the main neurotransmitter used by the parasympathetic nervous system). Atropine dilates the pupils, increases heart rate, and reduces salivation and other secretions [1].
References:
[1]. http://en.wikipedia.org/wiki/Atropine
- McN-A 343
Catalog No.:BCC7042
CAS No.:55-45-8
- Epinephrine HCl
Catalog No.:BCC4319
CAS No.:55-31-2
- Benzamide
Catalog No.:BCN5737
CAS No.:55-21-0
- HYOSCINE HYDROCHLORIDE
Catalog No.:BCN8331
CAS No.:55-16-3
- 3,3'',5-Triiodo-L-thyronine Sodium Salt
Catalog No.:BCN1419
CAS No.:55-06-1
- Shikonin acetyl
Catalog No.:BCN2452
CAS No.:54984-93-9
- Physalin D
Catalog No.:BCN7919
CAS No.:54980-22-2
- Tamoxifen Citrate
Catalog No.:BCC4382
CAS No.:54965-24-1
- Albendazole
Catalog No.:BCC3718
CAS No.:54965-21-8
- Florilenalin
Catalog No.:BCN6422
CAS No.:54964-49-7
- 2-Oxopomolic acid
Catalog No.:BCN5732
CAS No.:54963-52-9
- Shikalkin
Catalog No.:BCC8359
CAS No.:54952-43-1
- 1-Phenylbiguanide hydrochloride
Catalog No.:BCC6870
CAS No.:55-57-2
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Angustifoline
Catalog No.:BCN3205
CAS No.:550-43-6
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
- Afrormosine
Catalog No.:BCN3312
CAS No.:550-79-8
- Lupanine
Catalog No.:BCN5736
CAS No.:550-90-3
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
- NAADP tetrasodium salt
Catalog No.:BCC7808
CAS No.:5502-96-5
- (±)-Cloprostenol sodium salt
Catalog No.:BCC7315
CAS No.:55028-72-3
- Isorhamnetin-3-O-neohespeidoside
Catalog No.:BCN1234
CAS No.:55033-90-4
Comparison of the effects of topical and systemic atropine sulfate on intraocular pressure and pupil diameter in the normal canine eye.[Pubmed:24428364]
Vet Ophthalmol. 2015 Jan;18(1):43-9.
OBJECTIVE: To compare the effects of topical 1% Atropine sulfate and systemic 0.1% Atropine sulfate on the intraocular pressure (IOP) and horizontal pupil diameter (HPD) in the canine eye. PROCEDURES: Four groups, each containing 10 dogs of varying age, breed, and sex were treated as follows: (i) One 30 muL drop of topical 1% Atropine sulfate was applied unilaterally in each dog, (ii) A control group, one drop of 0.9% saline was used, (iii) 0.06 mg/kg Atropine sulfate was given by intramuscular injection, and (iv) Control with saline injected intramuscularly. In all groups, IOP and HPD were measured every 5 min over 60 min. RESULTS: Topical atropine significantly increased IOP in the treated eye with no change in the untreated eye. A maximum increase in IOP from 17.7 +/- 3.1 to 20.3 +/- 3.1 mmHg (14.7% increase) was obtained 23.0 +/- 14.3 min post-treatment. Maximal HPD of 12.1 +/- 1.7 mm in the treated eye occurred 46.5 +/- 6.3 min after treatment, with no increase in the untreated eye. Systemic atropine caused an increase in IOP in both eyes, showing a maximum at 15.5 +/- 10.6 min post-treatment with an IOP of 17.3 +/- 4.6 mmHg in the right eye and 17.1 +/- 5.2 mmHg in the left eye (21.8% increase in the right eye and 21.6% in the left eye). Maximal HPD was noted in both eyes 30.0 +/- 11.6 min after treatment. CONCLUSIONS: Atropine sulfate causes a significant increase in IOP when given both topically and by intramuscular injection. It should be used with caution, or indeed avoided entirely, in dogs with glaucoma or in those with a predisposition to the condition.
Formulation and characterization of atropine sulfate in albumin-chitosan microparticles for in vivo ocular drug delivery.[Pubmed:25652269]
J Pharm Sci. 2015 May;104(5):1677-90.
The overall study goal was to produce a microparticle formulation containing Atropine sulfate for ocular administration with improved efficacy and lower side effects, compared with that of the standard marketed atropine solution. The objective was to prepare an Atropine sulfate-loaded bovine serum albumin-chitosan microparticle that would have longer contact time on the eyes as well as better mydriatic and cycloplegic effect using a rabbit model. The microparticle formulation was prepared by method of spray-drying technique. The percent drug loading and encapsulation efficiency were assessed using a USP (I) dissolution apparatus. The particle sizes and zeta potential were determined using laser scattering technique and the surface morphology of the microparticles was determined using a scanning electron microscope. The product yield was calculated from relative amount of material used. In vitro cytotoxicity and uptake by human corneal epithelial cells were examined using AlamarBlue and confocal microscopy. The effects of the microparticle formulation on mydriasis in comparison with the marketed Atropine sulfate solution were evaluated in rabbit eyes. The prepared microparticle formulation had ideal physicochemical characteristics for delivery into the eyes. The in vivo studies showed that the microparticles had superior effects on mydriasis in rabbits than the marketed solutions


