TorilinCAS# 13018-10-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13018-10-5 | SDF | Download SDF |
| PubChem ID | 6450226 | Appearance | Powder |
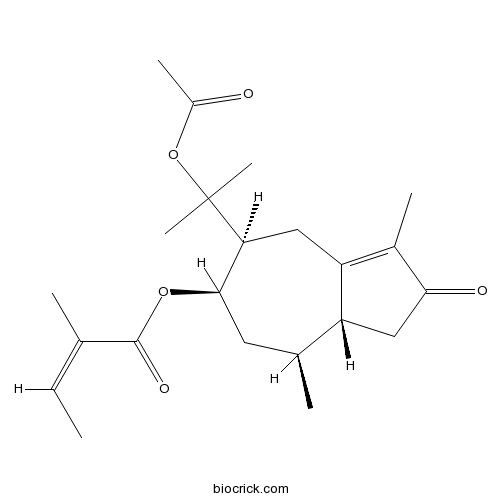
| Formula | C22H32O5 | M.Wt | 376.5 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(5S,6R,8S,8aR)-5-(2-acetyloxypropan-2-yl)-3,8-dimethyl-2-oxo-4,5,6,7,8,8a-hexahydro-1H-azulen-6-yl] (Z)-2-methylbut-2-enoate | ||
| SMILES | CC=C(C)C(=O)OC1CC(C2CC(=O)C(=C2CC1C(C)(C)OC(=O)C)C)C | ||
| Standard InChIKey | IQWVFAXBJQKUDH-TXCQZRSTSA-N | ||
| Standard InChI | InChI=1S/C22H32O5/c1-8-12(2)21(25)26-20-9-13(3)16-11-19(24)14(4)17(16)10-18(20)22(6,7)27-15(5)23/h8,13,16,18,20H,9-11H2,1-7H3/b12-8-/t13-,16+,18-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Torilin has anti-inflammatory properties, it inhibits inflammation by limiting TAK1-mediated MAP kinase and NF-κB activation. 2. Torilin can attenuate arthritis severity, modify leukocyte activations in dLNs or joints, and restore serum and splenocyte cytokine imbalances, it may have immunomodulatory and anti-inflammatory properties with the capacity to ameliorate the inflammatory response in collagen-induced-arthritis-(CIA)-mice. 3. Torilin inhibits melanin production in alpha-melanocyte stimulating hormone-activated B16 melanoma cells, with an IC(50) value of 25 microM. 4. Torilin shows excellent antimicrobial activity against Bacillus subtilis ATCC 6633 spores and vegetative cells, it functions as a surfactant with hydrophilic and hydrophobic properties related to denaturalization of various proteins,the distortion of coat proteins due to direct binding polar groups of spore coats with hydrophilic groups of torilin may be responsible for the observed rapid inactivation of bacterial spores. 5. Torilin has a potent anti-angiogenic activity both in vivo and in vitro, and it may have a strong activity to suppress tumorigenesis by inhibition of tumor invasion. 6. Torilin is an inhibitor of testosterone 5 alpha-reductase, it (IC50 = 31.7 +/- 4.23 microM) shows a stronger inhibition of 5 alpha-reductase than alpha-linolenic acid (IC50 = 160.3 +/- 24.62 microM) but is weaker than finasteride (IC50 = 0.38 +/- 0.06 microM). 7. Torilin reverses multidrug-resistance in cancer cells, it can potentiate the cytotoxicities of adriamycin, vinblastine, taxol and colchicine against multidrug-resistant KB-V1 and MCF7/ADR cells. 8. Torilin and torilolone show hepatoprotective effects on tacrine-induced cytotoxicity in human liver-derived Hep G2 cells, the EC50 values are 20.6 +/- 1.86 and 3.6 +/- 0.1 microM, respectively; and silybin as a positive control shows an EC50 value of 69.0 +/- 3.4 microM. |
| Targets | p38MAPK | JNK | ERK | AP-1 | p65 | NF-kB | IkB | Tyrosinase | MMP(e.g.TIMP) | 5-alpha Reductase | IKK |

Torilin Dilution Calculator

Torilin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.656 mL | 13.2802 mL | 26.5604 mL | 53.1208 mL | 66.4011 mL |
| 5 mM | 0.5312 mL | 2.656 mL | 5.3121 mL | 10.6242 mL | 13.2802 mL |
| 10 mM | 0.2656 mL | 1.328 mL | 2.656 mL | 5.3121 mL | 6.6401 mL |
| 50 mM | 0.0531 mL | 0.2656 mL | 0.5312 mL | 1.0624 mL | 1.328 mL |
| 100 mM | 0.0266 mL | 0.1328 mL | 0.2656 mL | 0.5312 mL | 0.664 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Torilolone
Catalog No.:BCN6659
CAS No.:13018-09-2
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Protopine
Catalog No.:BCN6165
CAS No.:130-86-9
- Thioridazine HCl
Catalog No.:BCC3869
CAS No.:130-61-0
- Cirsimarin
Catalog No.:BCN6821
CAS No.:13020-19-4
- N-(4-Hydroxyphenylacetyl)spermine
Catalog No.:BCC6594
CAS No.:130210-32-1
- Acerogenin G
Catalog No.:BCN7328
CAS No.:130233-83-9
- 3'-Demethoxypiplartine
Catalog No.:BCN4021
CAS No.:130263-10-4
- PD 135158
Catalog No.:BCC7431
CAS No.:130285-87-9
- Rubiarbonol B
Catalog No.:BCN6159
CAS No.:130288-60-7
- Fmoc-Asp(OcHex)-OH
Catalog No.:BCC3468
CAS No.:130304-80-2
- HOE 140
Catalog No.:BCC5964
CAS No.:130308-48-4
- Fmoc-D-Tic-OH
Catalog No.:BCC3342
CAS No.:130309-33-0
- Fmoc-Oic-OH
Catalog No.:BCC3305
CAS No.:130309-37-4
- H-D-Phe-OMe.HCl
Catalog No.:BCC3013
CAS No.:13033-84-6
- CI 988
Catalog No.:BCC7430
CAS No.:130332-27-3
Anti-invasive activity of torilin, a sesquiterpene compound isolated from Torilis japonica.[Pubmed:11182056]
Oncol Rep. 2001 Mar-Apr;8(2):359-64.
Torilin is a sesquiterpene compound purified from Torilis japonica (Umbelliferae). We have previously reported that Torilin has a potent anti-angiogenic activity both in vivo and in vitro. In the present study, we investigated the anti-invasive activity of Torilin, and interestingly found that Torilin completely blocked intravasation of HT1080 human fibrosarcoma cells inoculated on the chorioallantoic membrane (CAM) of chick embryo. In addition, Torilin decreased the attachment of HT1080 cells to confluent human umbilical vein endothelial cells (HUVECs) at non-toxic concentration. In in vitro transwell invasion model, 25 microM Torilin also significantly inhibited HT1080 cell invasion in a time-dependent manner. Activity and expression of matrix metalloproteinase-9 (MMP-9) that is very important in tumor invasion and metastasis were also decreased by Torilin treatment, indicating that the inhibitory effect of Torilin on invasion of HT1080 cells may result from decreasing activity and expression of MMP-9. Therefore, it is possible that Torilin may decrease metastatic potential of tumor cells through inhibiting their attachment to endothelial cells and intravasation to blood vessels. Taken together, Torilin may have a strong activity to suppress tumorigenesis by inhibition of tumor invasion.
Antimicrobial activity of torilin isolated from Torilis japonica fruit against Bacillus subtilis.[Pubmed:18298734]
J Food Sci. 2008 Mar;73(2):M37-46.
Torilis japonica fruit has been used in therapeutic antimicrobial treatments in Korea and China since ancient times, but there is still little information on the mechanism underlying this activity. We found that the ethanol extract of T. japonica fruit showed excellent antimicrobial activity against Bacillus subtilis ATCC 6633 spores and vegetative cells. The crude ethanol extract (75%) reduced the spore concentration by 3 log cycles and the vegetative cell concentration to lower than the detection level (reduction in spore concentration by more than 6 orders of magnitude) at a concentration of 1% (w/v). The ethanol extract of T. japonica fruit was fractionated into n-hexane (H) and a water layer. The active antimicrobial compound was isolated and purified from the hexane layer, and identified as Torilin (5-[1-(acetyloxy)-1-methylethyl]-3,8-dimethyl-2-oxo-1,2,4,5,6,7,8,8a-octa-hydroaz ulen-6-yl(2E)-2-methylbut-2-enoate; C(22)H(32)O(5)). Torilin immediately reduced vegetative cells counts by 5 to 6 orders of magnitude, and reduced spores counts by 1 order of magnitude. The integrity of structures such as the inner, middle, and outer layers of the coat and the cortex, protoplast membrane, and core are vital to spores. Torilin functions as a surfactant with hydrophilic and hydrophobic properties related to denaturalization of various proteins. The distortion of coat proteins due to direct binding polar groups of spore coats with hydrophilic groups of Torilin may be responsible for the observed rapid inactivation of bacterial spores.
Sesquiterpenes with hepatoprotective activity from Cnidium monnieri on tacrine-induced cytotoxicity in Hep G2 cells.[Pubmed:12221602]
Planta Med. 2002 Aug;68(8):748-9.
Bioassay-guided fractionation of the EtOH extract of Cnidium monnieri (Apiaceae) furnished two hepatoprotective sesquiterpenes, Torilin (1) and torilolone (2), together with a new derivative, 1-hydroxyTorilin (3). Compounds 1 and 2 showed hepatoprotective effects on tacrine-induced cytotoxicity in human liver-derived Hep G2 cells. The EC50 values of compounds 1 and 2 were 20.6 +/- 1.86 (P < 0.01) and 3.6 +/- 0.1 (P < 0.01) microM, respectively. Silybin as a positive control showed an EC50 value of 69.0 +/- 3.4 microM.
Torilin from Torilis japonica inhibits melanin production in alpha-melanocyte stimulating hormone-activated B16 melanoma cells.[Pubmed:19533579]
Planta Med. 2009 Nov;75(14):1505-8.
Epidermal melanocytes synthesize melanin pigments and transfer them to keratinocytes, which is responsible for skin pigmentation. However, abnormal accumulation of melanin pigments causes hyperpigmentation disorders, which are substantially improved with treatment of tyrosinase inhibitor. In our ongoing study, Torilis japonica DC. (Umbelliferae) was found to inhibit melanin production. A goal of this study is to elucidate the hypopigmenting principle of T. japonica. A sesquiterpene structure of Torilin was isolated from the plant extracts via bioassay-guided phytochemical analysis. Torilin dose-dependently inhibited melanin production, with an IC(50) value of 25 microM, in alpha-melanocyte stimulating hormone (alpha-MSH)-activated B16 melanoma cells. Arbutin, a positive control of skin whitener, also inhibited alpha-MSH-induced melanin production with an IC(50) value of 170 microM. As to the mode of action, Torilin downregulated alpha-MSH-induced protein levels of tyrosinase without directly inhibiting catalytic activity of the enzyme. Taken together, this study shows that Torilin contributes to the hypopigmenting principle of T. japonica, and suggests its pharmacological potential in melanin-associated hyperpigmentation disorders.
Torilin from Torilis japonica, as a new inhibitor of testosterone 5 alpha-reductase.[Pubmed:12802730]
Planta Med. 2003 May;69(5):459-61.
The methanolic extract of the fruits of Torilis japonica showed a potent inhibition against 5 alpha-reductase activity in vitro. Bioassay-guided fractionation of the methanol extract of the fruits followed by repeated silica gel chromatography led to the isolation of an active principle and its structure was identified as Torilin on the basis of spectroscopic data. Torilin (IC50 = 31.7 +/- 4.23 microM) showed a stronger inhibition of 5 alpha-reductase than alpha-linolenic acid (IC50 = 160.3 +/- 24.62 microM) but was weaker than finasteride. (IC50 = 0.38 +/- 0.06 microM). Simple guaiane-type compounds, such as (-)-guaiol and guaiazulene showed weak inhibitory effects on the 5 alpha-reductase activity with IC50 values of f 81.6 microM and 100.8 microM, respectively, while azulene was not active. These results suggest that both degrees of unsaturation and the side-chain in the guaiane skeleton are important for the manifestation of 5 alpha-reductase inhibition.
Torilin Inhibits Inflammation by Limiting TAK1-Mediated MAP Kinase and NF-kappaB Activation.[Pubmed:28316375]
Mediators Inflamm. 2017;2017:7250968.
Torilin, a sesquiterpene isolated from the fruits of Torilis japonica, has shown antimicrobial, anticancer, and anti-inflammatory properties. However, data on the mechanism of Torilin action against inflammation is limited. This study aimed at determining the anti-inflammatory property of Torilin in LPS-induced inflammation using in vitro model of inflammation. We examined Torilin's effect on expression levels of inflammatory mediators and cytokines in LPS-stimulated RAW 264.7 macrophages. The involvement of NF-kB and AP-1, MAP kinases, and adaptor proteins were assessed. Torilin strongly inhibited LPS-induced NO release, iNOS, PGE2, COX-2, NF-alpha, IL-1beta, IL-6, and GM-CSF gene and protein expressions. In addition, MAPKs were also suppressed by Torilin pretreatment. Involvement of ERK1/2, P38(MAPK), and JNK1/2 was further confirmed by PD98059, SB203580, and SP600125 mediated suppression of iNOS and COX-2 proteins. Furthermore, Torilin attenuated NF-kB and AP-1 translocation, DNA binding, and reporter gene transcription. Interestingly, Torilin inhibited TAK1 kinase activation with the subsequent suppression of MAPK-mediated JNK, p38, ERK1/2, and AP-1 (ATF-2 and c-jun) activation and IKK-mediated I-kappaBalpha degradation, p65/p50 activation, and translocation. Together, the results revealed the suppression of NF-kappaB and AP-1 regulated inflammatory mediator and cytokine expressions, suggesting the test compound's potential as a candidate anti-inflammatory agent.
Torilin ameliorates type II collagen-induced arthritis in mouse model of rheumatoid arthritis.[Pubmed:23623942]
Int Immunopharmacol. 2013 Jun;16(2):232-42.
Advancements in rheumatoid-arthritis-(RA) therapies have shown considerable progresses in the comprehension of disease. However, the development of new potential agents with relative safety and efficacy continues and natural compounds have been considered as alternatives to identify new entities. Since previous in-vivo data and our in-vitro findings showed that Torilin has a strong anti-inflammatory property, we further investigated its effect against collagen-induced-arthritis-(CIA) in mice. CIA-induced DBA/1J mice were treated with Torilin or methotrexate (MTX) for 5-weeks. Arthritis severity was evaluated by arthritic score and joint histopathology. Draining lymph node (dLN), joint and peripheral-blood mononuclear-cell (PBMC) counts, and activation/localization of T-/B-lymphocytes, dendritic cells (DCs) and neutrophils were examined by FACS analysis. Serum anti-type-II-collagen-(CII) antibody levels and cultured-splenocyte and serum cytokines were also evaluated. Torilin markedly reduced CIA-induced arthritic score, histopathology and leukocyte counts. Besides, Torilin suppressed CIA-activated T-cells including CD3+, CD3+/CD69+, CD8+, CD4+ and CD4+/CD25+ in dLNs or joints. It also modified CD19+ or CD20+/CD23+ (B-cells), MHCII+/CD11c+ (DCs) and Gr-1+/CD11b+ (neutrophil) subpopulations. It further depressed total anti-CII-IgG, anti-CII-IgG1 and anti-CII-IgG2a antibody productions. Moreover, while IFN-gamma and IL-10 were not affected, Torilin suppressed CIA-induced serum TNF-alpha, IL-1beta and IL-6 levels. Interestingly, Torilin also blocked IFN-gamma, IL-17 and IL-6 cytokines while it did not affect IL-10 but enhanced IL-4 in splenocytes. These results show that Torilin attenuated arthritis severity, modified leukocyte activations in dLNs or joints, and restored serum and splenocyte cytokine imbalances. Torilin may have immunomodulatory and anti-inflammatory properties with the capacity to ameliorate the inflammatory response in CIA-mice.
Torilin, a sesquiterpene from Torilis japonica, reverses multidrug-resistance in cancer cells.[Pubmed:9619115]
Planta Med. 1998 May;64(4):332-4.
A sesquiterpene compound reversing multidrug-resistance in cancer cells was isolated from the fruits of Torilis japonica and the structure was identified as Torilin. Torilin potentiated the cytotoxicities of adriamycin, vinblastine, taxol and colchicine against multidrug-resistant KB-V1 and MCF7/ADR cells.


