ProtopineCAS# 130-86-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130-86-9 | SDF | Download SDF |
| PubChem ID | 4970 | Appearance | White powder |
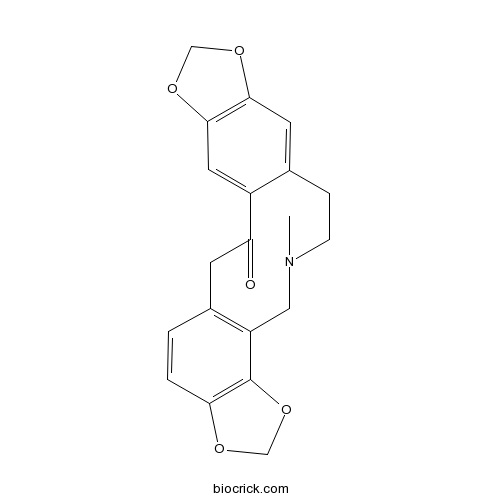
| Formula | C20H19NO5 | M.Wt | 353.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Corydinine | ||
| Solubility | DMSO : 12.5 mg/mL (35.37 mM; Need ultrasonic) | ||
| SMILES | CN1CCC2=CC3=C(C=C2C(=O)CC4=C(C1)C5=C(C=C4)OCO5)OCO3 | ||
| Standard InChIKey | GPTFURBXHJWNHR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19NO5/c1-21-5-4-13-7-18-19(25-10-24-18)8-14(13)16(22)6-12-2-3-17-20(15(12)9-21)26-11-23-17/h2-3,7-8H,4-6,9-11H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protopine acts as a potent inhibitor of thromboxane synthesis and PAF with hepatoprotective, antidepressant, antioxidant, antispasmodic and relaxant properties. Protopine is also a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines. Protopine blocks phosphorylation of mitogen-activated protein kinases (MAP kinases) and also blocks activation of a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). |
| Targets | COX | EGFR | Integrin | Calcium Channel | Caspase | NF-kB | 5-HT Receptor | CDK | Bcl-2/Bax | PGE | NO |
| In vitro | Protopine inhibits heterotypic cell adhesion in MDA-MB-231 cells through down-regulation of multi-adhesive factors.[Pubmed: 25435628]Afr. J. Tradit. Complement. Altern. Med., 2014 Jan 28;11(2):415-24.A Chinese herb Corydalis yanhusuo W.T. Wang that showed anticancer and anti-angiogenesis effects in our previous studies was presented for further studies. In the present study, we studied the anticancer proliferation and adhesion effects of five alkaloids which were isolated from Corydalis yanhusuo.
|
| In vivo | Anti-thrombotic and anti-inflammatory activities of protopine.[Pubmed: 9368908 ]Pharmacol Res. 1997 Jul;36(1):1-7.
Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine.[Pubmed: 18164606 ]Phytomedicine. 2008 Jun;15(6-7):470-7.
Antispasmodic and relaxant activity of chelidonine, protopine, coptisine, and Chelidonium majus extracts on isolated guinea-pig ileum.[Pubmed: 9933996 ]Planta Med. 1998 Dec;64(8):758-60.
|
| Kinase Assay | Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines.[Pubmed: 22033245 ]Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages.[Pubmed: 22360889]BMB Rep. 2012 Feb;45(2):108-13.Protopine is an isoquinoline alkaloid contained in plants in northeast Asia. Cancer Lett. 2012 Feb 1;315(1):1-11.
|
| Cell Research | Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms.[Pubmed: 18602385 ]Eur J Pharmacol. 2008 Sep 4;591(1-3):21-7.Calcium and lipid peroxidation play important roles in oxidative stress-induced cellular injury and apoptosis, which ultimately cause cell death. |
| Animal Research | Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models.[Pubmed: 16530230]Neuropharmacology. 2006 Jun;50(8):934-40.The Protopine isolated from a Chinese herb Dactylicapnos scandens Hutch was identified as an inhibitor of both serotonin transporter and noradrenaline transporter in vitro assays. |

Protopine Dilution Calculator

Protopine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8297 mL | 14.1483 mL | 28.2965 mL | 56.5931 mL | 70.7414 mL |
| 5 mM | 0.5659 mL | 2.8297 mL | 5.6593 mL | 11.3186 mL | 14.1483 mL |
| 10 mM | 0.283 mL | 1.4148 mL | 2.8297 mL | 5.6593 mL | 7.0741 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.5659 mL | 1.1319 mL | 1.4148 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.5659 mL | 0.7074 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Protopine, an isoquinoline alkaloid contained in plants in northeast Asia. IC50 Value: Target: In vitro: Protopine was found to reduce nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E(2) (PGE(2)) production by LPS-stimulated Raw 264.7 cells, without a cytotoxic effect. Pre-treatment of Raw 264.7 cells with protopine reduced the production of pro-inflammatory cytokines [2]. Protopine is a novel microtubule stabilizer with anticancer activity in HRPC cells through apoptotic pathway by modulating Cdk1 activity and Bcl-2 family of proteins [3]. In HepG2 cells, protopine significantly increased CYP1A1 mRNA levels after 24h exposure at concentrations from 25 and 10 μM. Protopine also dose-dependently increased CYP1A1 and CYP1A2 mRNA levels in human hepatocytes [4]. In vivo: Assays were performed on MDA-MB-231 human breast cancer cells, and the result showed that protopine exhibited anti-adhesive and anti-invasion effects in MDA-MB-231 cells; after treatment with protopine for 90 min, the expression of EGFR, ICAM-1, αv-integrin, β1-integrin and β5-integrin were remarkably reduced [1].
References:
[1]. Bae DS, et al. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep. 2012 Feb;45(2):108-13.
[2]. Chen CH, et al. Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines. Cancer Lett. 2012 Feb 1;315(1):1-11.
[3]. Vrba J, et al. Protopine and allocryptopine increase mRNA levels of cytochromes P450 1A in human hepatocytes and HepG2 cells independently of AhR. Toxicol Lett. 2011 Jun 10;203(2):135-41.
[4]. He K, et al. Protopine inhibits heterotypic cell adhesion in MDA-MB-231 cells through down-regulation of multi-adhesive factors. Afr J Tradit Complement Altern Med. 2014 Jan 28;11(2):415-24.
- Thioridazine HCl
Catalog No.:BCC3869
CAS No.:130-61-0
- 1,4-Naphthoquinone
Catalog No.:BCN8420
CAS No.:130-15-4
- Senecionine
Catalog No.:BCN2129
CAS No.:130-01-8
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Dapoxetine HCl
Catalog No.:BCC5064
CAS No.:129938-20-1
- Amicarbazone
Catalog No.:BCC5464
CAS No.:129909-90-6
- MC 976
Catalog No.:BCC1734
CAS No.:129831-99-8
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
- Anemoside B4
Catalog No.:BCN1276
CAS No.:129741-57-7
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
- Torilolone
Catalog No.:BCN6659
CAS No.:13018-09-2
- Torilin
Catalog No.:BCN6611
CAS No.:13018-10-5
- Cirsimarin
Catalog No.:BCN6821
CAS No.:13020-19-4
Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models.[Pubmed:16530230]
Neuropharmacology. 2006 Jun;50(8):934-40.
The Protopine isolated from a Chinese herb Dactylicapnos scandens Hutch was identified as an inhibitor of both serotonin transporter and noradrenaline transporter in vitro assays. 5-hydroxy-DL-tryptophan(5-HTP)-induced head twitch response (HTR) and tail suspension test were adopted to study whether Protopine has anti-depression effect in mice using reference antidepressant fluoxetine and desipramine as positive controls. In HTR test, Protopine at doses of 5, 10, 20 mg/kg dose dependently increase the number of 5-HTP-induced HTR. Protopine at doses of 3.75 mg/kg, 7.5 mg/kg and 30 mg/kg also produces a dose-dependent reduction in immobility in the tail suspension test. The present results open up new possibilities for the use of Protopine in the treatment of mood disorders, such as mild and moderate states of depression.
Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines.[Pubmed:22033245]
Cancer Lett. 2012 Feb 1;315(1):1-11.
In this study, we investigated the anticancer effect of Protopine on human hormone-refractory prostate cancer (HRPC) cells. Protopine exhibited an anti-proliferative effect by induction of tubulin polymerization and mitotic arrest, which ultimately led to apoptotic cell death. The data suggest that Protopine increased the activity of cyclin-dependent kinase 1 (Cdk1)/cyclin B1 complex and that contributed to cell apoptosis by modulating mitochondria-mediated signaling pathways, such as Bcl-2 phosphorylation and Mcl-1 down-regulation. In conclusion, the data suggest that Protopine is a novel microtubule stabilizer with anticancer activity in HRPC cells through apoptotic pathway by modulating Cdk1 activity and Bcl-2 family of proteins.
Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine.[Pubmed:18164606]
Phytomedicine. 2008 Jun;15(6-7):470-7.
The present investigation demonstrates the hepatoprotective potential of 50% ethanolic water extract of whole plant of Fumaria indica and its three fractions viz., hexane, chloroform and butanol against d-galactosamine induced hepatotoxicity in rats. The hepatoprotection was assessed in terms reduction in histological damage, changes in serum enzymes (SGOT, SGPT, ALP) and metabolites bilirubin, reduced glutathione (GSH) and lipid peroxidation (MDA content). Among fractions more than 90% protection was found with butanol fraction in which alkaloid Protopine was quantified as highest i.e. about 0.2mg/g by HPTLC. The isolated Protopine in doses of 10-20mg p.o. also proved equally effective hepatoprotectants as standard drug silymarine (single dose 25mg p.o.). In general all treatments excluding hexane fraction proved hepatoprotective at par with silymarine (p Calcium and lipid peroxidation play important roles in oxidative stress-induced cellular injury and apoptosis, which ultimately cause cell death. In this study we examined whether Protopine had a neuroprotection against H(2)O(2)-induced injury in PC12 cells. Pretreatment of PC12 cells with Protopine improved the cell viability, enhanced activities of superoxide dismutase, glutathione peroxidase and catalase, and decreased malondialdehyde level in the H(2)O(2) injured cells. Protopine also reversed the increased intracellular Ca(2+) concentration and the reduced mitochondrial membrane potential caused by H(2)O(2) in the cells. Furthermore, Protopine was able to inhibit caspase-3 expression and cell apoptosis induced by H(2)O(2). In summary, this study demonstrates that Protopine is able to relieve H(2)O(2)-induced oxidative stress and apoptosis in PC12 cells, at least in part, by Ca(2+) antagonism and antioxidant mechanisms. BACKGROUND: A Chinese herb Corydalis yanhusuo W.T. Wang that showed anticancer and anti-angiogenesis effects in our previous studies was presented for further studies. In the present study, we studied the anticancer proliferation and adhesion effects of five alkaloids which were isolated from Corydalis yanhusuo. MATERIALS AND METHODS: MTT dose response curves, cell migration assay, cell invasion assay, as well as three types of cell adhesive assay were performed on MDA-MB-231 human breast cancer cells. The mechanism of the compounds on inhibiting heterotypic cell adhesion were further explored by determining the expression of epidermal growth factor receptor (EGFR), Intercellular adhesion molecule 1 (ICAM-1), alphav-integrin, beta1-integrin and beta5-integrin by western blotting assay. RESULTS: In five tested alkaloids, only Protopine exhibited anti-adhesive and anti-invasion effects in MDA-MB-231 cells, which contributed to the anti-metastasis effect of Corydalis yanhusuo. The results showed that after treatment with Protopine for 90 min, the expression of EGFR, ICAM-1, alphav-integrin, beta1-integrin and beta5-integrin were remarkably reduced. CONCLUSION: The present results suggest that Protopine seems to inhibit the heterotypic cell adhesion between MDA-MB-231 cells, and human umbilical vein endothelial cells by changing the expression of adhesive factors. The effects of Protopine on human platelet aggregation and arachidonic acid (AA) metabolism via cyclooxygenase (COX) and lipoxygenase (LOP) enzymes were examined. Platelet aggregation induced by various platelet agonists (AA, ADP, collagen and PAF) was strongly inhibited by Protopine in a concentration-related manner. The IC50 values (microM) of Protopine (mean +/- SEM) against: AA; 12 +/- 2: ADP; 9 +/- 2: collagen; 16 +/- 2 and PAF; 11 +/- 1, were much less than those observed for aspirin. In addition, Protopine selectively inhibited the synthesis of thromboxane A2 (TXA2) via COX pathway and had no effect on the LOP pathway in platelets. In vivo, pretreatment with Protopine (50-100 mg kg-1) protected rabbits from the lethal effects of AA (2 mg kg-1) or PAF (11 micrograms kg-1) in dose-dependent fashion. Protopine (50-100 mg kg-1) also inhibited carrageenan-induced rat paw oedema with a potency of three-fold as compared to aspirin. These results are suggestive that Protopine acts as a potent inhibitor of thromboxane synthesis and PAF with anti-inflammatory properties. Two ethanolic dry extracts from the herb Chelidonium majus L. with a defined content of the main alkaloids (chelidonine, Protopine, and coptisisine) and the alkaloids themselves were studied in three different antispasmodic test models on isolated ileum of guinea-pigs. In the BaCl2-stimulated ileum, chelidonine and Protopine exhibited the known papaverine-like musculotropic action, whereas coptisine (up to 3.0 x 10(-5) g/ml) was ineffective in this model. Both extracts were active with 53.5% and 49.0% relaxation at 5 x 10(-4) g/ml. The carbachol and the electric field stimulated contractions were antagonized by all three alkaloids. Coptisine showed competitive antagonist behaviour with a pA2 value of 5.95. Chelidonine and Protopine exhibited a certain degree of non-competitive antagonism. In the electric field the antagonist activities decreased in the order Protopine > coptisine > chelidonine. The concentrations of the chelidonium herb extracts for 50% inhibition of the carbachol and electrical field induced spasms were in the range of 2.5 to 5 x 10(-4) g/ml. Protopine is an isoquinoline alkaloid contained in plants in northeast Asia. In this study, we investigated whether Protopine derived from Hypecoum erectum L could suppress lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (Raw 264.7 cells). Protopine was found to reduce nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E(2) (PGE(2)) production by LPS-stimulated Raw 264.7 cells, without a cytotoxic effect. Pre-treatment of Raw 264.7 cells with Protopine reduced the production of pro-inflammatory cytokines. These inhibitory effects were caused by blocking phosphorylation of mitogen-activated protein kinases (MAP kinases) and also blocking activation of a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB).Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms.[Pubmed:18602385]
Eur J Pharmacol. 2008 Sep 4;591(1-3):21-7.
Protopine inhibits heterotypic cell adhesion in MDA-MB-231 cells through down-regulation of multi-adhesive factors.[Pubmed:25435628]
Afr J Tradit Complement Altern Med. 2014 Jan 28;11(2):415-24. eCollection 2014.
Anti-thrombotic and anti-inflammatory activities of protopine.[Pubmed:9368908]
Pharmacol Res. 1997 Jul;36(1):1-7.
Antispasmodic and relaxant activity of chelidonine, protopine, coptisine, and Chelidonium majus extracts on isolated guinea-pig ileum.[Pubmed:9933996]
Planta Med. 1998 Dec;64(8):758-60.
Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages.[Pubmed:22360889]
BMB Rep. 2012 Feb;45(2):108-13.


