Thioridazine HClCalcium channel protein inhibitor CAS# 130-61-0 |
- Lomeguatrib
Catalog No.:BCC1133
CAS No.:192441-08-0
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130-61-0 | SDF | Download SDF |
| PubChem ID | 66062 | Appearance | Powder |
| Formula | C21H27ClN2S2 | M.Wt | 407 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 75 mM in water and to 100 mM in DMSO | ||
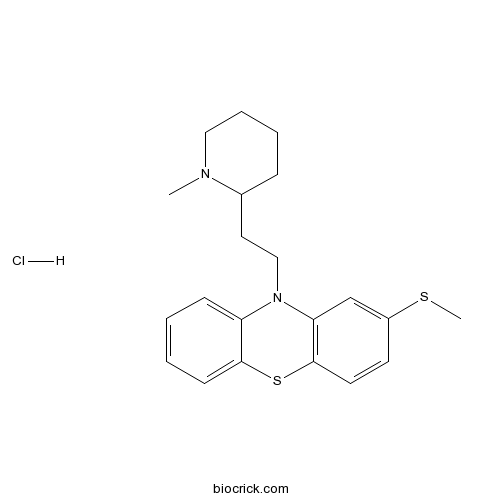
| Chemical Name | 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine;hydrochloride | ||
| SMILES | CN1CCCCC1CCN2C3=CC=CC=C3SC4=C2C=C(C=C4)SC.Cl | ||
| Standard InChIKey | NZFNXWQNBYZDAQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H26N2S2.ClH/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23;/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine receptor antagonist that displays antipsychotic activity. Exhibits anticancer activity in cervical, endometrial and breast cancer cells; triggers apoptosis by targeting the PI 3-K/Akt/mTOR/p70 S6K pathway. Induces G1 cell cycle arrest. Selective inducer of cancer stem cell (CSC) differentiation. | |||||

Thioridazine HCl Dilution Calculator

Thioridazine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.457 mL | 12.285 mL | 24.57 mL | 49.14 mL | 61.4251 mL |
| 5 mM | 0.4914 mL | 2.457 mL | 4.914 mL | 9.828 mL | 12.285 mL |
| 10 mM | 0.2457 mL | 1.2285 mL | 2.457 mL | 4.914 mL | 6.1425 mL |
| 50 mM | 0.0491 mL | 0.2457 mL | 0.4914 mL | 0.9828 mL | 1.2285 mL |
| 100 mM | 0.0246 mL | 0.1229 mL | 0.2457 mL | 0.4914 mL | 0.6143 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Thioridazine·HCl is a calcium channel protein inhibitor as well as D2DR/D4DR inhibitor. Its administration has been associated with hypotension. It has also been, after chronic treatment, used to test increased sensitivity of dopaminergic agents.
- 1,4-Naphthoquinone
Catalog No.:BCN8420
CAS No.:130-15-4
- Senecionine
Catalog No.:BCN2129
CAS No.:130-01-8
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Dapoxetine HCl
Catalog No.:BCC5064
CAS No.:129938-20-1
- Amicarbazone
Catalog No.:BCC5464
CAS No.:129909-90-6
- MC 976
Catalog No.:BCC1734
CAS No.:129831-99-8
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
- Anemoside B4
Catalog No.:BCN1276
CAS No.:129741-57-7
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- Protopine
Catalog No.:BCN6165
CAS No.:130-86-9
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
- Torilolone
Catalog No.:BCN6659
CAS No.:13018-09-2
- Torilin
Catalog No.:BCN6611
CAS No.:13018-10-5
Flame atomic absorption determination of palladium in solutions after preconcentration using octadecyl silica membrane disks modified by thioridazine.HCl.[Pubmed:18969890]
Talanta. 2005 Feb 28;65(4):925-9.
A new simple and reliable method for rapid and selective extraction and determination of trace level of Pd(II) ion is developed. Palladium ions are adsorbed quantitatively during passage of aqueous samples through octadecyl silica membrane disks modified with thioridazine.HCl (TRH). The influence of flow rates of eluent and sample solution, amount of ligand, types and least amount of eluent, and pH of samples were studied. Almost all matrix elements were found to pass through the disk to drain. Break through volume and limit of detection of the membrane disks modified by 5mg of TRH was found to be 1.0 l and 12mugl(-1), respectively. The retained Pd(II) ions are then stripped from the disk with a minimal amount of sulfite solution as eluent and subsequently measured by atomic absorption spectrometry. The proposed method permitted large enrichment factors of about 100 and higher. The method was applied to the recovery of Pd(II) ions from different industrial samples and waters.
A comparison of the cardiovascular effects of haloperidol, thioridazine and chlorpromazine HCl.[Pubmed:6251766]
Arch Int Pharmacodyn Ther. 1980 Mar;244(1):48-57.
Transient hypotension, electrocardiographic conduction changes and cardiac arrhythmias have been reported with the use of certain anti-psychotic agents. To assess these observations, we compared the effects of haloperidol, thioridazine and chlorpromazine HCl on alpha-adrenergic receptors, mean aortic pressure, myocardial contractile force and myocardial electrophysiology in anesthetized dogs. The results from these studies indicated that, on a milligram basis, chlorpromazine HCl was equipotent to haloperidol, and thioridazine was 0.5 times as potent as haloperidol as an alpha-adrenergic receptor blocker and as a myocardial depressant. Each compound induced conduction nonhomogeneity in myocardial conducting tissue. However, as a neuroleptic, when tested in dogs, haloperidol was 50 times more potent than chlorpromazine HCl and 180 times more potent than thioridazine. The therapeutic safety indices (cardiovascular effect dose/neuroleptic dose) for haloperidol regarding alpha-adrenergic blockade, hypotension, myocardial depression and ventricular conduction delay were 4, 145, 235 and 125, respectively, whereas these indices for chlorpromazine and for thioridazine were 0.06, 2, 4, 6 and 0.05, 1, 3 and 1, respectively. Therefore, because of differences in neuroleptic potency, therapeutic doses of haloperidol are less likely to cause adverse cardiovascular effects than are those of either chlorpromazine HCl or thioridazine.
Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.[Pubmed:22460505]
Apoptosis. 2012 Sep;17(9):989-97.
Recently, thioridazine (10-[2-(1-methyl-2-piperidyl) ethyl]-2-methylthiophenothiazine), a well-known anti-psychotic agent was found to have anti-cancer activity in cancer cells. However, the molecular mechanism of the agent in cellular signal pathways has not been well defined. Thioridazine significantly increased early- and late-stage apoptotic fraction in cervical and endometrial cancer cells, suggesting that suppression of cell growth by thioridazine was due to the induction of apoptosis. Cell cycle analysis indicated thioridazine induced the down-regulation of cyclin D1, cyclin A and CDK4, and the induction of p21 and p27, a cyclin-dependent kinase inhibitor. Additionally, we compared the influence of thioridazine with cisplatin used as a control, and similar patterns between the two drugs were observed in cervical and endometrial cancer cell lines. Furthermore, as expected, thioridazine successfully inhibited phosphorylation of Akt, phosphorylation of 4E-BP1 and phosphorylation of p70S6K, which is one of the best characterized targets of the mTOR complex cascade. These results suggest that thioridazine effectively suppresses tumor growth activity by targeting the PI3K/Akt/mTOR/p70S6K signaling pathway.
Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells.[Pubmed:22632761]
Cell. 2012 Jun 8;149(6):1284-97.
Selective targeting of cancer stem cells (CSCs) offers promise for a new generation of therapeutics. However, assays for both human CSCs and normal stem cells that are amenable to robust biological screens are limited. Using a discovery platform that reveals differences between neoplastic and normal human pluripotent stem cells (hPSC), we identify small molecules from libraries of known compounds that induce differentiation to overcome neoplastic self-renewal. Surprisingly, thioridazine, an antipsychotic drug, selectively targets the neoplastic cells, and impairs human somatic CSCs capable of in vivo leukemic disease initiation while having no effect on normal blood SCs. The drug antagonizes dopamine receptors that are expressed on CSCs and on breast cancer cells as well. These results suggest that dopamine receptors may serve as a biomarker for diverse malignancies, demonstrate the utility of using neoplastic hPSCs for identifying CSC-targeting drugs, and provide support for the use of differentiation as a therapeutic strategy.


