4-Methylcinnamic acidCAS# 1866-39-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1866-39-3 | SDF | Download SDF |
| PubChem ID | 731767 | Appearance | White cryst. |
| Formula | C10H10O2 | M.Wt | 162.19 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
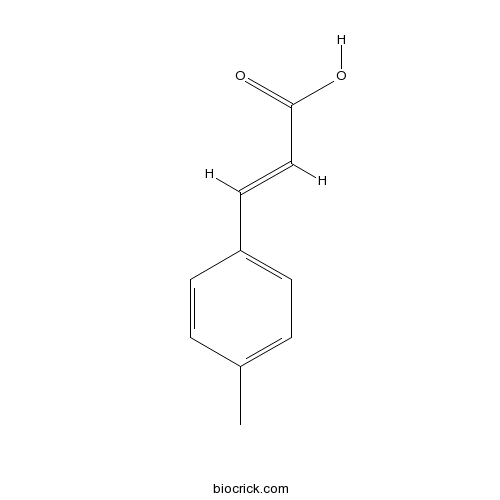
| Chemical Name | (E)-3-(4-methylphenyl)prop-2-enoic acid | ||
| SMILES | CC1=CC=C(C=C1)C=CC(=O)O | ||
| Standard InChIKey | RURHILYUWQEGOS-VOTSOKGWSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Methylcinnamic acid is a photosensitive compound. |
| Structure Identification | Journal of Raman Spectroscopy.1998 April;29(4):263–267.Crystalline state photoreaction in 4-methylcinnamic acid: a Raman phonon spectroscopic study[Reference: WebLink]The solid-state photodimerization reaction mechanism in 4-Methylcinnamic acid crystal was investigated. Raman and infrared spectroscopy were used to study the intramolecular vibrations of the reactant and the product in order to characterize them. US 8758864 B2[P]. 2014.Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same[Reference: WebLink]4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-chlorocinnamic acid, 2-methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-Methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(trifluoromethyl)-cinnamic acid, 4-chlorocinnamic acid, 4-bromocinnamic acid, 4-methoxycinnamic acid, 4-hydroxycinnamic acid, 4-nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene. |

4-Methylcinnamic acid Dilution Calculator

4-Methylcinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.1656 mL | 30.828 mL | 61.6561 mL | 123.3122 mL | 154.1402 mL |
| 5 mM | 1.2331 mL | 6.1656 mL | 12.3312 mL | 24.6624 mL | 30.828 mL |
| 10 mM | 0.6166 mL | 3.0828 mL | 6.1656 mL | 12.3312 mL | 15.414 mL |
| 50 mM | 0.1233 mL | 0.6166 mL | 1.2331 mL | 2.4662 mL | 3.0828 mL |
| 100 mM | 0.0617 mL | 0.3083 mL | 0.6166 mL | 1.2331 mL | 1.5414 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Allyl cinnamate
Catalog No.:BCC8812
CAS No.:1866-31-5
- 1,2-Bis(3-indenyl)ethane
Catalog No.:BCC8413
CAS No.:18657-57-3
- LY 344864
Catalog No.:BCC1716
CAS No.:186544-26-3
- Zibotentan (ZD4054)
Catalog No.:BCC2524
CAS No.:186497-07-4
- Alisol B
Catalog No.:BCN3364
CAS No.:18649-93-9
- Actein
Catalog No.:BCN1159
CAS No.:18642-44-9
- Psoralidin
Catalog No.:BCN5414
CAS No.:18642-23-4
- CP 316819
Catalog No.:BCC6039
CAS No.:186392-43-8
- CP-91149
Catalog No.:BCC3757
CAS No.:186392-40-5
- enantio-7(11)-Eudesmen-4-ol
Catalog No.:BCN1158
CAS No.:186374-63-0
- H-Dapa-OH.HBr
Catalog No.:BCC2668
CAS No.:18635-45-5
- (-)-Anonaine
Catalog No.:BCN8235
CAS No.:1862-41-5
- 2-NBDG
Catalog No.:BCC6530
CAS No.:186689-07-6
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- H-D-Tyr(tBu)-OH
Catalog No.:BCC3137
CAS No.:186698-58-8
- Ketamine hydrochloride
Catalog No.:BCC5982
CAS No.:1867-66-9
- N6-methyladenosine (m6A)
Catalog No.:BCC6495
CAS No.:1867-73-8
- Alisol A 24-acetate
Catalog No.:BCN2344
CAS No.:18674-16-3
- Ginsenoside Rg5
Catalog No.:BCN3551
CAS No.:186763-78-0
- ML 10302 hydrochloride
Catalog No.:BCC7695
CAS No.:186826-17-5
- Moxifloxacin HCl
Catalog No.:BCC2507
CAS No.:186826-86-8
- 2B-(SP)
Catalog No.:BCC5817
CAS No.:186901-17-7
- Pafuramidine
Catalog No.:BCC1832
CAS No.:186953-56-0
- Sinapine
Catalog No.:BCN1815
CAS No.:18696-26-9
Probing the Crystal Structure Landscape by Doping: 4-Bromo, 4-Chloro, and 4-Methylcinnamic Acids.[Pubmed:29893027]
Angew Chem Int Ed Engl. 2018 Jul 20;57(30):9279-9283.
Accessing the data points in the crystal structure landscape of a molecule is a challenging task, either experimentally or computationally. We have charted the crystal structure landscape of 4-bromocinnamic acid (4BCA) experimentally and computationally: experimental doping is achieved with 4-Methylcinnamic acid (4MCA) to obtain new crystal structures; computational doping is performed with 4-chlorocinnamic acid (4CCA) as a model system, because of the difficulties associated in parameterizing the Br atom. The landscape of 4CCA is explored experimentally in turn, also by doping it with 4MCA, and is found to bear a close resemblance to the landscape of 4BCA, justifying the ready miscibility of these two halogenated cinnamic acids to form solid solutions without any change in crystal structure. In effect, 4MCA, 4CCA and 4BCA form a commutable group of crystal structures, which may be realized experimentally or computationally, and constitute the landscape. Unlike the results obtained by Kitaigorodskii, all but two of the multiple solid solutions obtained in the methyl-doping experiments take structures that are different from the hitherto observed crystal forms of the parent compounds. Even granted that the latter might be inherently polymorphic, this unusual observation provokes the suggestion that solid solution formation may be used to probe the crystal structure landscape. The influence of pipi interactions, weak hydrogen bonds and halogen bonds in directing the formation of these new structures is also seen.
Cinnamic Acid Analogs as Intervention Catalysts for Overcoming Antifungal Tolerance.[Pubmed:29065462]
Molecules. 2017 Oct 21;22(10). pii: molecules22101783.
Disruption of fungal cell wall should be an effective intervention strategy. However, the cell wall-disrupting echinocandin drugs, such as caspofungin (CAS), cannot exterminate filamentous fungal pathogens during treatment. For potency improvement of cell wall-disrupting agents (CAS, octyl gallate (OG)), antifungal efficacy of thirty-three cinnamic acid derivatives was investigated against Saccharomyces cerevisiaeslt2Delta, bck1Delta, mutants of the mitogen-activated protein kinase (MAPK), and MAPK kinase kinase, respectively, in cell wall integrity system, and glr1Delta, mutant of CAS-responsive glutathione reductase. Cell wall mutants were highly susceptible to four cinnamic acids (4-chloro-alpha-methyl-, 4-methoxy-, 4-methyl-, 3-methylcinnamic acids), where 4-chloro-alpha-methyl- and 4-Methylcinnamic acids possessed the highest activity. Structure-activity relationship revealed that 4-Methylcinnamic acid, the deoxygenated structure of 4-methoxycinnamic acid, overcame tolerance of glr1Delta to 4-methoxycinnamic acid, indicating the significance of para substitution of methyl moiety for effective fungal control. The potential of compounds as chemosensitizers (intervention catalysts) to cell wall disruptants (viz., 4-chloro-alpha-methyl- or 4-Methylcinnamic acids + CAS or OG) was assessed according to Clinical Laboratory Standards Institute M38-A. Synergistic chemosensitization greatly lowers minimum inhibitory concentrations of the co-administered drug/agents. 4-Chloro-alpha-methylcinnamic acid further overcame fludioxonil tolerance of Aspergillus fumigatus antioxidant MAPK mutants (sakADelta, mpkCDelta). Collectively, 4-chloro-alpha-methyl- and 4-Methylcinnamic acids possess chemosensitizing capability to augment antifungal efficacy of conventional drug/agents, thus could be developed as target-based (i.e., cell wall disruption) intervention catalysts.
Crystal engineering: co-crystals of cinnamic acid derivatives with a pyridyl derivative co-crystallizer.[Pubmed:26830799]
Acta Crystallogr B Struct Sci Cryst Eng Mater. 2016 Feb;72(Pt 1):87-95.
A number of hydrogen-bonded co-crystals, consisting of a cinnamic acid derivative and a pyridyl co-crystallizer, have been synthesized and their properties investigated by X-ray diffraction. Samples were prepared by recrystallization or solvent drop grinding of trans-cinnamic acid (1), 4-Methylcinnamic acid (2), 4-methoxy cinnamic acid (3) or 3,4-methoxy cinnamic acid (4), with 4,4-dipyridyl (A), iso-nicotinamide (B) or nicotinamide (C). The X-ray single-crystal structures of seven novel co-crystals, obtained through recrystallization, are examined and the hydrogen-bonding interactions discussed. Consistent hydrogen-bonding motifs were observed for samples prepared when using 4,4-dipyridyl (A) or iso-nicotinamide (B) as the co-crystallizing agent. Powder X-ray diffraction analysis of the samples prepared by solvent drop grinding suggests the formation of ten co-crystals.


