Zibotentan (ZD4054)ETA receptor antagonist,potent and specific CAS# 186497-07-4 |
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 186497-07-4 | SDF | Download SDF |
| PubChem ID | 9910224 | Appearance | Powder |
| Formula | C19H16N6O4S | M.Wt | 424.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (58.90 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
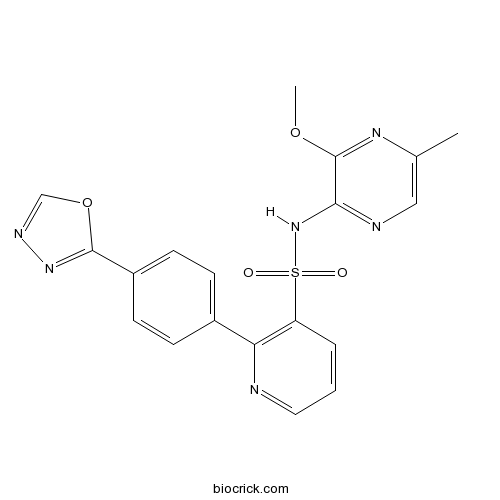
| Chemical Name | N-(3-methoxy-5-methylpyrazin-2-yl)-2-[4-(1,3,4-oxadiazol-2-yl)phenyl]pyridine-3-sulfonamide | ||
| SMILES | CC1=CN=C(C(=N1)OC)NS(=O)(=O)C2=C(N=CC=C2)C3=CC=C(C=C3)C4=NN=CO4 | ||
| Standard InChIKey | FJHHZXWJVIEFGJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H16N6O4S/c1-12-10-21-17(19(23-12)28-2)25-30(26,27)15-4-3-9-20-16(15)13-5-7-14(8-6-13)18-24-22-11-29-18/h3-11H,1-2H3,(H,21,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Zibotentan (ZD4054) is a specific antagonist of Endothelin (ET)A with an IC50 value of 21 nM. | |||||
| Targets | ETA | |||||
| IC50 | 21 nM | |||||

Zibotentan (ZD4054) Dilution Calculator

Zibotentan (ZD4054) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3561 mL | 11.7805 mL | 23.561 mL | 47.122 mL | 58.9025 mL |
| 5 mM | 0.4712 mL | 2.3561 mL | 4.7122 mL | 9.4244 mL | 11.7805 mL |
| 10 mM | 0.2356 mL | 1.1781 mL | 2.3561 mL | 4.7122 mL | 5.8903 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4712 mL | 0.9424 mL | 1.1781 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4712 mL | 0.589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ZD4054 is a specific antagonist of the endothelin A receptor.
The autocrine endothelin (ET)-1/endothelin A receptor (ETAR) pathway is an important regulator of several processes involved in ovarian cancer progression.
In vitro: In the human ovarian cancer ETAR positive cell lines, ZD4054 effectively inhibited the basal and ET-1-induced cell proliferation, with the inhibition of AKT and p42/44MAPK phosphorylation, and increased apoptosis, through the inhibition of bcl-2 and activation of caspase-3 and poly(ADP-ribose) polymerase proteins. [1].
In vivo: In HEY ovarian cancer xenografts, ZD4054 inhibited tumor growth to the same degree as paclitaxel. Moreover, ZD4054-dependent tumor growth inhibition was associated with a reduction in proliferation index, MMP-2 expression, and microvessel microvessel density [2].
Clinical trial: The PK and safety profiles of ZD4054 determined in this Chinese patient population are similar to those previously reported. Findings suggest no clinically relevant inter-ethnic differences in ZD4054 disposition between the patient populations analyzed [3].
References:
[1] Morris CD, Rose A, Curwen J, Hughes AM, Wilson DJ, Webb DJ. Specific inhibition of the endothelin A receptor with ZD4054: clinical and pre-clinical evidence. Br J Cancer. 2005 Jun 20;92(12):2148-52.
[2] Rosanò L, Di Castro V, Spinella F, Nicotra MR, Natali PG, Bagnato A. ZD4054, a specific antagonist of the endothelin A receptor, inhibits tumor growth and enhances paclitaxel activity in human ovarian carcinoma in vitro and in vivo. Mol Cancer Ther. 2007 Jul;6(7):2003-11.
[3] Li J, Liu Y, Qian J, Wu L, Kemp J, Nii M, Tomkinson H, Zuo Y, Ranson M, Usami M. Single- and multiple-dose pharmacokinetics, safety and tolerability of zibotentan (ZD4054) in Chinese men with advanced solid tumors. Cancer Chemother Pharmacol. 2012 Jul;70(1):57-63.
- Alisol B
Catalog No.:BCN3364
CAS No.:18649-93-9
- Actein
Catalog No.:BCN1159
CAS No.:18642-44-9
- Psoralidin
Catalog No.:BCN5414
CAS No.:18642-23-4
- CP 316819
Catalog No.:BCC6039
CAS No.:186392-43-8
- CP-91149
Catalog No.:BCC3757
CAS No.:186392-40-5
- enantio-7(11)-Eudesmen-4-ol
Catalog No.:BCN1158
CAS No.:186374-63-0
- H-Dapa-OH.HBr
Catalog No.:BCC2668
CAS No.:18635-45-5
- (-)-Anonaine
Catalog No.:BCN8235
CAS No.:1862-41-5
- 11alpha,12alpha-Epoxy-3beta,23-dihydroxy-30-norolean-20(29)-en-28,13beta-olide
Catalog No.:BCN1515
CAS No.:186140-36-3
- Quercetin-3-O-sophoroside
Catalog No.:BCN2771
CAS No.:18609-17-1
- Deacetylnimbin
Catalog No.:BCN4684
CAS No.:18609-16-0
- Kakuol
Catalog No.:BCN6455
CAS No.:18607-90-4
- LY 344864
Catalog No.:BCC1716
CAS No.:186544-26-3
- 1,2-Bis(3-indenyl)ethane
Catalog No.:BCC8413
CAS No.:18657-57-3
- Allyl cinnamate
Catalog No.:BCC8812
CAS No.:1866-31-5
- 4-Methylcinnamic acid
Catalog No.:BCN5034
CAS No.:1866-39-3
- 2-NBDG
Catalog No.:BCC6530
CAS No.:186689-07-6
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- H-D-Tyr(tBu)-OH
Catalog No.:BCC3137
CAS No.:186698-58-8
- Ketamine hydrochloride
Catalog No.:BCC5982
CAS No.:1867-66-9
- N6-methyladenosine (m6A)
Catalog No.:BCC6495
CAS No.:1867-73-8
- Alisol A 24-acetate
Catalog No.:BCN2344
CAS No.:18674-16-3
- Ginsenoside Rg5
Catalog No.:BCN3551
CAS No.:186763-78-0
- ML 10302 hydrochloride
Catalog No.:BCC7695
CAS No.:186826-17-5
Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer.[Pubmed:23381694]
Prostate Cancer Prostatic Dis. 2013 Jun;16(2):187-92.
BACKGROUND: Standard treatment options are limited for the management of non-metastatic castration-resistant prostate cancer (CRPC). This study, part of the ENTHUSE (EndoTHelin A USE) phase III programme, evaluated the efficacy and safety of the oral specific endothelin (ET)A receptor antagonist zibotentan vs placebo in patients with non-metastatic CRPC (non-mCRPC). METHODS: This was a multicentre, randomized, double-blind, phase III study. Patients (n=1421) with non-mCRPC and biochemical progression (determined by rising serum PSA levels) were randomized to receive zibotentan 10 mg or placebo once daily. Based on the lack of efficacy signal in another ENTHUSE phase III study, an interim analysis was performed to determine whether the study was likely to achieve the co-primary objectives of improved overall survival (OS) and progression-free survival (PFS). RESULTS: Criteria for continuation of this study were not met. A total of 79 deaths and 293 progression events were recorded at final data cutoff. Zibotentan-treated patients did not significantly differ from placebo-treated patients for OS (hazard ratio (HR): 1.13; 95% confidence interval (CI): 0.73-1.76, P=0.589) or PFS (HR: 0.89; 95% CI: 0.71-1.12, P=0.330). The most commonly reported adverse events in zibotentan-treated patients were peripheral oedema (37.7%), headache (26.2%) and nasal congestion (24.9%); each occurred with >15% higher incidence than in the placebo group. CONCLUSIONS: This trial was terminated early because of failure at interim analysis of the efficacy data to meet the defined criteria for continuation. Owing to the absence of demonstrable survival benefits in the ENTHUSE clinical studies, zibotentan is no longer under investigation as a potential treatment for prostate cancer.
Efficacy of the specific endothelin a receptor antagonist zibotentan (ZD4054) in colorectal cancer: a preclinical study.[Pubmed:23723122]
Mol Cancer Ther. 2013 Aug;12(8):1556-67.
Endothelin 1 (ET-1) is overexpressed in cancer, contributing to disease progression. We previously showed that ET-1 stimulated proliferative, migratory, and contractile tumorigenic effects via the ET(A) receptor. Here, for the first time, we evaluate zibotentan, a specific ET(A) receptor antagonist, in the setting of colorectal cancer, in cellular models. Pharmacologic characteristics were further determined in patient tissues. Colorectal cancer lines (n = 4) and fibroblast strains (n = 6), isolated from uninvolved areas of colorectal cancer specimens, were exposed to ET-1 and/or ET(A)/(B) receptor antagonists. Proliferation (methylene blue), migration (scratch wounds), and contraction (gel lattices) were assessed. Receptor distribution and binding characteristics (K(d), B(max)) were determined using autoradiography on tissue sections and homogenates and cytospun cells, supported by immunohistochemistry. Proliferation was inhibited by ET(A) (zibotentan > BQ123; P < 0.05), migration by ET(B) > ET(A), and contraction by combined ET(A) and ET(B) antagonism. Intense ET-1 stromal binding correlated with fibroblasts and endothelial cells. Colorectal cancer lines and fibroblasts revealed high density and affinity ET-1 binding (B(max) = 2.435 fmol/1 x 10(6) cells, K(d) = 367.7 pmol/L; B(max) = 3.03 fmol/1 x 10(6) cells, K(d) = 213.6 pmol/L). In cancer tissues, ET(A) receptor antagonists (zibotentan; BQ123) reduced ET-1 binding more effectively (IC(50): 0.1-10 mumol/L) than ET(B) receptor antagonist BQ788 ( approximately IC(50), 1 mmol/L). ET-1 stimulated cancer-contributory processes. Its localization to tumor stroma, with greatest binding/affinity to fibroblasts, implicates these cells in tumor progression. ET(A) receptor upregulation in cancer tissues and its role in tumorigenic processes show the receptor's importance in therapeutic targeting. Zibotentan, the most specific ET(A) receptor antagonist available, showed the greatest inhibition of ET-1 binding. With its known safety profile, we provide evidence for zibotentan's potential role as adjuvant therapy in colorectal cancer.
Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone.[Pubmed:22786751]
Cancer. 2012 Nov 15;118(22):5709-18.
BACKGROUND: Endothelin-1 and the endothelin A (ET(A) ) receptor have been implicated in prostate cancer progression in bone. This study aimed to determine whether the specific ET(A) receptor antagonist, zibotentan, prolonged overall survival (OS) in patients with castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain. METHODS: Patients were randomized 1:1 to zibotentan 10 mg/day or placebo, plus standard prostate cancer treatment. The primary endpoint was OS. Secondary endpoints included times to pain progression, chemotherapy use, new bone metastases, and safety. Efficacy endpoints were analyzed using a log-rank test. RESULTS: A total of 594 patients were randomized (zibotentan, n = 299; placebo, n = 295). Median OS was 24.5 months in zibotentan-treated patients versus 22.5 months for placebo, but the difference did not reach statistical significance (hazard ratio, 0.87; 95.2% confidence interval, 0.69-1.10; P = .240). No statistically significant differences were observed for any secondary efficacy endpoints. Peripheral edema (44%) and headache (31%) were the most commonly reported adverse events in the zibotentan group. Cardiac failure events were higher in the zibotentan group than placebo (any grade, 5.7% and 1.7%; Common Terminology Criteria for Adverse Events grade >/=3, 3.0% and 1.0%, respectively); these were manageable and reversible. CONCLUSIONS: In this large, randomized, placebo-controlled phase 3 trial, treatment with zibotentan 10 mg/day did not lead to a statistically significant improvement in OS in this patient population. Zibotentan had an acceptable safety profile.
A Phase II, randomized, double-blind study of zibotentan (ZD4054) in combination with carboplatin/paclitaxel versus placebo in combination with carboplatin/paclitaxel in patients with advanced ovarian cancer sensitive to platinum-based chemotherapy (AGO-OVAR 2.14).[Pubmed:23234805]
Gynecol Oncol. 2013 Jul;130(1):31-7.
BACKGROUND: In platinum-sensitive relapsed ovarian cancer, paclitaxel plus carboplatin is a standard second-line treatment. Zibotentan (ZD4054) is an oral, specific ETA-receptor antagonist with demonstrated antitumour activity in xenograft models of human ovarian cancer. METHODS: In this Phase II, randomized, placebo-controlled study, patients with relapsed ovarian cancer sensitive to platinum-based chemotherapy received zibotentan 10mg or placebo once-daily, plus paclitaxel 175 mg/m(2) iv followed by carboplatin iv (AUC 5) on day 1 of every 3-week cycle for a maximum of eight cycles. The primary endpoint was progression-free survival (PFS), evaluated by Response Evaluation Criteria In Solid Tumours (RECIST). Secondary and exploratory endpoints included objective tumour response rate, tumour size, CA-125/RECIST progression, and safety and tolerability. RESULTS: A total of 120 patients were randomized (zibotentan: n=59; placebo: n=61). Addition of zibotentan 10mg/day to carboplatin and paclitaxel did not improve PFS compared with placebo (median PFS, 7.6 versus 10.0 months, respectively; HR=1.46, [80% CI: 1.10-1.94]; P=0.0870). No improvements in any of the secondary or exploratory efficacy endpoints were observed for patients receiving zibotentan compared with placebo. Median duration of total treatment exposure was 6.7 months. Total chemotherapy dose received was lower for zibotentan-treated versus placebo-treated patients (carboplatin: -16%; paclitaxel: -14%). The most common adverse events in the zibotentan arm were anaemia, nausea, alopecia, headache and neutropenia (43-48% of patients). CONCLUSIONS: Zibotentan 10mg/day plus carboplatin and paclitaxel did not result in an improvement in PFS compared with chemotherapy alone in patients with advanced ovarian cancer sensitive to platinum-based chemotherapy. No unexpected safety concerns were identified.


