KakuolCAS# 18607-90-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18607-90-4 | SDF | Download SDF |
| PubChem ID | 596894 | Appearance | Powder |
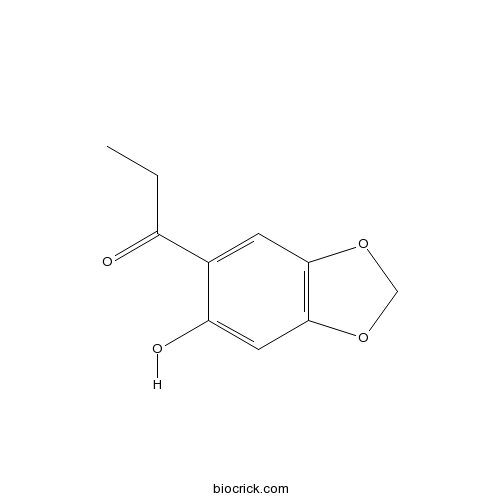
| Formula | C10H10O4 | M.Wt | 194.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(6-hydroxy-1,3-benzodioxol-5-yl)propan-1-one | ||
| SMILES | CCC(=O)C1=CC2=C(C=C1O)OCO2 | ||
| Standard InChIKey | SLLMHZXMVHNZOR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H10O4/c1-2-7(11)6-3-9-10(4-8(6)12)14-5-13-9/h3-4,12H,2,5H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kakuol has antifungal activity, it can completely inhibit the mycelial growth of Botrytis cinerea Pers ex Fr and Cladosporium cucumerinum Ellis & Arthur at 50 microg ml(-1) and 30 microg ml(-1), respectively. 2. kakuol and a derivative analogue are able to inhibit the DNA relaxation mediated by the human enzyme. |
| Targets | Topoisomerase | Antifection |

Kakuol Dilution Calculator

Kakuol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1493 mL | 25.7467 mL | 51.4933 mL | 102.9866 mL | 128.7333 mL |
| 5 mM | 1.0299 mL | 5.1493 mL | 10.2987 mL | 20.5973 mL | 25.7467 mL |
| 10 mM | 0.5149 mL | 2.5747 mL | 5.1493 mL | 10.2987 mL | 12.8733 mL |
| 50 mM | 0.103 mL | 0.5149 mL | 1.0299 mL | 2.0597 mL | 2.5747 mL |
| 100 mM | 0.0515 mL | 0.2575 mL | 0.5149 mL | 1.0299 mL | 1.2873 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RS 79948 hydrochloride
Catalog No.:BCC6876
CAS No.:186002-54-0
- AMD 3465
Catalog No.:BCC5181
CAS No.:185991-24-6
- AMD 3465 hexahydrobromide
Catalog No.:BCC5182
CAS No.:185991-07-5
- H-Ile-OMe.HCl
Catalog No.:BCC2964
CAS No.:18598-74-8
- H-Cys-OMe.HCl
Catalog No.:BCC2905
CAS No.:18598-63-5
- Echinulin
Catalog No.:BCN1157
CAS No.:1859-87-6
- Pterodontic acid
Catalog No.:BCN1156
CAS No.:185845-89-0
- 2,3-Dihydroxypterodontic acid
Catalog No.:BCN1155
CAS No.:185821-32-3
- Viroallosecurinine
Catalog No.:BCN6743
CAS No.:1857-30-3
- BMS 961
Catalog No.:BCC7680
CAS No.:185629-22-5
- ONO 2506
Catalog No.:BCC7943
CAS No.:185517-21-9
- Timorsaponin C
Catalog No.:BCN2899
CAS No.:185432-00-2
- Deacetylnimbin
Catalog No.:BCN4684
CAS No.:18609-16-0
- Quercetin-3-O-sophoroside
Catalog No.:BCN2771
CAS No.:18609-17-1
- 11alpha,12alpha-Epoxy-3beta,23-dihydroxy-30-norolean-20(29)-en-28,13beta-olide
Catalog No.:BCN1515
CAS No.:186140-36-3
- (-)-Anonaine
Catalog No.:BCN8235
CAS No.:1862-41-5
- H-Dapa-OH.HBr
Catalog No.:BCC2668
CAS No.:18635-45-5
- enantio-7(11)-Eudesmen-4-ol
Catalog No.:BCN1158
CAS No.:186374-63-0
- CP-91149
Catalog No.:BCC3757
CAS No.:186392-40-5
- CP 316819
Catalog No.:BCC6039
CAS No.:186392-43-8
- Psoralidin
Catalog No.:BCN5414
CAS No.:18642-23-4
- Actein
Catalog No.:BCN1159
CAS No.:18642-44-9
- Alisol B
Catalog No.:BCN3364
CAS No.:18649-93-9
- Zibotentan (ZD4054)
Catalog No.:BCC2524
CAS No.:186497-07-4
A derivative of the natural compound kakuol affects DNA relaxation of topoisomerase IB inhibiting the cleavage reaction.[Pubmed:23262316]
Arch Biochem Biophys. 2013 Feb 1;530(1):7-12.
Topoisomerases IB are anticancer and antimicrobial targets whose inhibition by several natural and synthetic compounds has been documented over the last three decades. Here we show that Kakuol, a natural compound isolated from the rhizomes of Asarum sieboldii, and a derivative analogue are able to inhibit the DNA relaxation mediated by the human enzyme. The analogue is the most efficient one and the inhibitory effect is enhanced upon pre-incubation with the enzyme. Analysis of the different steps of the catalytic cycle indicates that the inhibition occurs at the cleavage level and does not prevent DNA binding. Molecular docking shows that the compound preferentially binds near the active site at the bottom of the catalytic residue Tyr723, providing an atomistic explanation for its inhibitory activity.
Isolation and antifungal activity of kakuol, a propiophenone derivative from Asarum sieboldii rhizome.[Pubmed:15846774]
Pest Manag Sci. 2005 Aug;61(8):821-5.
An antifungal substance active against Colletotrichum orbiculare (Berk & Mont) Arx was isolated from the methanol extracts of Asarum sieboldii (Miq) Maek rhizomes. High-resolution MS, NMR and UV spectral data confirmed that the antifungal substance is Kakuol, 2-hydroxy-4,5-methylenedioxypropiophenone. Colletotrichum orbiculare was most sensitive to Kakuol, with MIC of 10 microg ml(-1). Kakuol also completely inhibited the mycelial growth of Botrytis cinerea Pers ex Fr and Cladosporium cucumerinum Ellis & Arthur at 50 microg ml(-1) and 30 microg ml(-1), respectively. However, no antimicrobial activity was found against yeast and bacteria even at 100 microg ml(-1). Kakuol exhibited a protective activity against the development of anthracnose disease on cucumber plants. The control efficacy of Kakuol against the anthracnose disease was in general somewhat less than that of the commercial fungicide chlorothalonil. This is the first report to demonstrate in vitro and in vivo antifungal activity of Kakuol against C. orbiculare infection.


