ONO 2506CAS# 185517-21-9 |
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Prucalopride
Catalog No.:BCC5055
CAS No.:179474-81-8
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- Desvenlafaxine Succinate
Catalog No.:BCC5048
CAS No.:386750-22-7
- Desvenlafaxine
Catalog No.:BCC5038
CAS No.:93413-62-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 185517-21-9 | SDF | Download SDF |
| PubChem ID | 208925 | Appearance | Powder |
| Formula | C11H22O2 | M.Wt | 186.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Arundic acid | ||
| Solubility | DMSO : 40 mg/mL (214.72 mM; Need ultrasonic) | ||
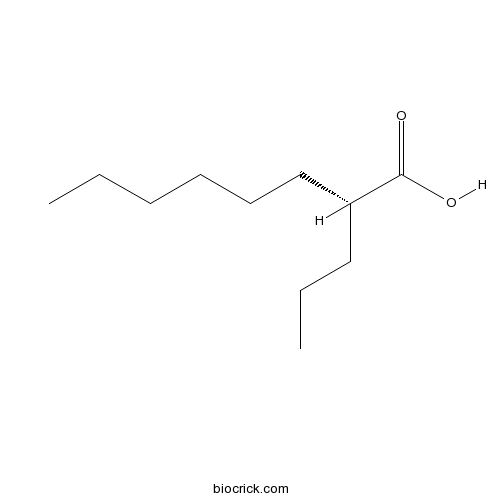
| Chemical Name | (2R)-2-propyloctanoic acid | ||
| SMILES | CCCCCCC(CCC)C(=O)O | ||
| Standard InChIKey | YCYMCMYLORLIJX-SNVBAGLBSA-N | ||
| Standard InChI | InChI=1S/C11H22O2/c1-3-5-6-7-9-10(8-4-2)11(12)13/h10H,3-9H2,1-2H3,(H,12,13)/t10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibits S-100β synthesis in activated cultured astrocytes. Prevents delayed infarct expansion 24 hours after permanent middle cerebral artery occlusion (pMCAO) in rats; also exhibits neuroprotective effects in mouse models of Parkinson's disease and Alzheimer's disease. |

ONO 2506 Dilution Calculator

ONO 2506 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.368 mL | 26.8399 mL | 53.6797 mL | 107.3595 mL | 134.1994 mL |
| 5 mM | 1.0736 mL | 5.368 mL | 10.7359 mL | 21.4719 mL | 26.8399 mL |
| 10 mM | 0.5368 mL | 2.684 mL | 5.368 mL | 10.7359 mL | 13.4199 mL |
| 50 mM | 0.1074 mL | 0.5368 mL | 1.0736 mL | 2.1472 mL | 2.684 mL |
| 100 mM | 0.0537 mL | 0.2684 mL | 0.5368 mL | 1.0736 mL | 1.342 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Timorsaponin C
Catalog No.:BCN2899
CAS No.:185432-00-2
- Corchoionoside C
Catalog No.:BCN1154
CAS No.:185414-25-9
- AF 12198
Catalog No.:BCC5812
CAS No.:185413-30-3
- O-Acetylethanolamine
Catalog No.:BCN1757
CAS No.:1854-30-4
- TCS 1105
Catalog No.:BCC6087
CAS No.:185391-33-7
- Fmoc-3-(2-Pyridyl)-Alanine
Catalog No.:BCC2568
CAS No.:185379-40-2
- Fmoc-3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2569
CAS No.:185379-39-9
- (R)-(+)-1,1'-Bi-2-naphthol
Catalog No.:BCC8393
CAS No.:18531-94-7
- GR 46611
Catalog No.:BCC5679
CAS No.:185259-85-2
- Rp-8-Br-PET-cGMPS
Catalog No.:BCC7538
CAS No.:185246-32-6
- Ceplignan
Catalog No.:BCN3626
CAS No.:185244-78-4
- Loganin
Catalog No.:BCN1153
CAS No.:18524-94-2
- BMS 961
Catalog No.:BCC7680
CAS No.:185629-22-5
- Viroallosecurinine
Catalog No.:BCN6743
CAS No.:1857-30-3
- 2,3-Dihydroxypterodontic acid
Catalog No.:BCN1155
CAS No.:185821-32-3
- Pterodontic acid
Catalog No.:BCN1156
CAS No.:185845-89-0
- Echinulin
Catalog No.:BCN1157
CAS No.:1859-87-6
- H-Cys-OMe.HCl
Catalog No.:BCC2905
CAS No.:18598-63-5
- H-Ile-OMe.HCl
Catalog No.:BCC2964
CAS No.:18598-74-8
- AMD 3465 hexahydrobromide
Catalog No.:BCC5182
CAS No.:185991-07-5
- AMD 3465
Catalog No.:BCC5181
CAS No.:185991-24-6
- RS 79948 hydrochloride
Catalog No.:BCC6876
CAS No.:186002-54-0
- Kakuol
Catalog No.:BCN6455
CAS No.:18607-90-4
- Deacetylnimbin
Catalog No.:BCN4684
CAS No.:18609-16-0
Immunohistochemical analysis of brain lesions using S100B and glial fibrillary acidic protein antibodies in arundic acid- (ONO-2506) treated stroke-prone spontaneously hypertensive rats.[Pubmed:19657585]
J Neural Transm (Vienna). 2009 Oct;116(10):1209-19.
Stroke-prone spontaneously hypertensive rats (SHRSP) used as a model of essential hypertension cause a high incidence of brain stroke on the course of hypertension. Incidences and sizes of brain lesions are known to relate to the astrocyte activities. Therefore, relation between brain damage and the expression profile of the astrocytes was investigated with morphometric and immunohistochemical analyses using astrocyte marker antibodies of S100B and glial fibrillary acidic protein (GFAP) with or without arundic acid administration, a suppressor on the activation of astrocytes. Arundic acid extended the average life span of SHRSP. An increase in brain tissue weight was inhibited concomitant with a lower rate of gliosis/hemosiderin deposit/scarring in brain lesions. S100B- or GFAP-positive dot and filamentous structures were decreased in arundic acid-treated SHRSP, and this effect was most pronounced in the cerebral cortex, white matter, and pons, and less so in the hippocampus, diencephalon, midbrain, and cerebellum. Blood pressure decreased after administration of arundic acid in the high-dose group (100 mg/kg/day arundic acid), but not in the low-dose group (30 mg/kg/day). These data indicate that arundic acid can prevent hypertension-induced stroke, and may inhibit the enlargement of the stroke lesion by preventing the inflammatory changes caused by overproduction of the S100B protein in the astrocytes.
ONO-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation.[Pubmed:22882023]
Br J Pharmacol. 2013 Mar;168(5):1088-100.
BACKGROUND AND PURPOSE: Anticonvulsants have been developed according to the traditional neurotransmission imbalance hypothesis. However, the anticonvulsive pharmacotherapy currently available remains unsatisfactory. To develop new antiepileptic drugs with novel antiepileptic mechanisms, we have tested the antiepileptic actions of ONO-2506, a glial modulating agent, and its effects on tripartite synaptic transmission. EXPERIMENTAL APPROACH: Dose-dependent effects of ONO-2506 on maximal-electroshock seizure (MES), pentylenetetrazol-induced seizure (PTZ) and epileptic discharge were determined in a genetic model of absence epilepsy in mice (Cacna1a(tm2Nobs/tm2Nobs) strain). Antiepileptic mechanisms of ONO-2506 were analysed by examining the interaction between ONO-2506 and transmission-modulating toxins (tetanus toxin, fluorocitrate, tetrodotoxin) on release of l-glutamate, d-serine, GABA and kynurenic acid in the medial-prefrontal cortex (mPFC) of freely moving rats using microdialysis and primary cultured rat astrocytes. KEY RESULTS: ONO-2506 inhibited spontaneous epileptic discharges in Cacna1a(tm2Nobs/tm2Nobs) mice without affecting MES or PTZ. Given systemically, ONO-2506 increased basal release of GABA and kynurenic acid in the mPFC through activation of both neuronal and glial exocytosis, but inhibited depolarization-induced releases of all transmitters. ONO-2506 increased basal glial release of kynurenic acid without affecting those of l-glutamate, d-serine or GABA. However, ONO-2506 inhibited AMPA-induced releases of l-glutamate, d-serine, GABA and kynurenic acid. CONCLUSIONS AND IMPLICATIONS: ONO-2506 did not affect traditional convulsive tests but markedly inhibited epileptic phenomena in the genetic epilepsy mouse model. ONO-2506 enhanced release of inhibitory neuro- and gliotransmitters during the resting stage and inhibited tripartite transmission during the hyperactive stage. The results suggest that ONO-2506 is a novel potential glial-targeting antiepileptic drug. LINKED ARTICLE: This article is commented on by Onat, pp. 1086-1087 of this issue. To view this commentary visit http://dx.doi.org/10.1111/bph.12050.
Arundic acid (ONO-2506) inhibits secondary injury and improves motor function in rats with spinal cord injury.[Pubmed:24360553]
J Neurol Sci. 2014 Feb 15;337(1-2):186-92.
BACKGROUND: Arundic acid (ONO-2506) inhibits the production and release of S100 protein from astrocytes. While numerous studies have assessed the effect of ONO-2506 in the diseased brain, to the best of our knowledge, no study has examined the effect of ONO-2506 in spinal cord injury (SCI). In this study, we administered ONO-2506 to rats with SCI in order to evaluate its effectiveness in improving motor function and protecting against histological injury. METHODS: All rats underwent laminectomy with SCI at the 10th thoracic vertebra. Rats were divided into 3 groups that received different concentrations of ONO-2506 as follows: 10 mg/kg (Group I) and 20 mg/kg (Group II). The third group (control group) was administered only saline. ONO-2506 or saline was administered by intravenous injection for a week after SCI. Recovery of motor function was assessed by determining the Basso, Beattie, and Bresnahan (BBB) scores and using the %grip test. Using immunohistochemistry, S100 protein and glial fibrillary acidic protein expression was assessed at week 12 post SCI. RESULTS: The BBB score of Group II was significantly better than that of the control group. At week 12 post SCI, the %grip was 43.0% in Group II and 20.3% in Group I. The score for the %grip test was greater for Group II than for the control group (7.0%); thus, motor function improvement appeared to be dose dependent. Regarding immunostaining evaluation, S100 protein staining was lower in Group II compared to the control group, and the astrocytic morphology resembled that of normal spinal cord sections. The SCI lesion expanded from the injured site to both proximal and distal sites in the control group and in Group I. However, despite the presence of cavitation, secondary expansion of the SCI lesion was prevented in Group II as a result of inhibition of S100 protein. CONCLUSIONS: Administration of ONO-2506 (20 mg/kg) improves motor function and inhibits expansion of secondary injury in SCI rats.
Arundic Acid ameliorates cerebral amyloidosis and gliosis in Alzheimer transgenic mice.[Pubmed:16709678]
J Pharmacol Exp Ther. 2006 Aug;318(2):571-8.
Like microglia, reactive astrocytes produce a myriad of neurotoxic substances in various brain pathologies, such as Alzheimer's disease (AD), trauma, and cerebral ischemia. Among the numerous products of reactive astrocytes, attention has recently been directed toward the possible detrimental role of S100B, because the protein has been shown to be highly expressed along with the progression of brain damage and to exert neurotoxic effects at high concentrations. The present study aimed to examine the possible role of astrocyte-derived S100B in the progression of cerebral amyloidosis and gliosis in transgenic mice overproducing mutant amyloid precursor protein (Tg APP(sw) mice, line 2576). For this purpose, arundic acid (Ono Pharmaceutical Co., Ltd., Mishima, Osaka, Japan), which is known to negatively regulate astrocyte synthesis of S100B, was orally administered to Tg APP(sw) mice for 6 months from 12 months of age, and the effects of the agent on the above parameters were examined. Here, we report that beta-amyloid deposits along with amyloid-beta peptide/S100B levels, as well as beta-amyloid plaque-associated reactive gliosis (astrocytosis and microgliosis), were significantly ameliorated in arundic acid-treated Tg APP(sw) mice relative to vehicle-treated Tg APP(sw) mice at 19 months of age. Based on the above results, arundic acid is considered to deserve further exploration as a promising therapeutic agent for AD.
Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice.[Pubmed:15567338]
Brain Res. 2004 Dec 24;1030(1):66-73.
We examined the neuroprotective effects of a novel astrocyte-modulating agent, arundic acid (ONO-2506), in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Male C57BL/6 mice received four intraperitoneal injections of MPTP (20 mg/kg) at 2 h intervals. Dopamine content in the striatum was reduced to 21% of the normal control after 7 days. Treatment with arundic acid (30 mg/kg, i.p.) administered 1 min, 6 h, 24 h, 48 h, and 72 h after the last MPTP injection prevented the dopamine depletion (52% of the control, p<0.01). In addition, this treatment resulted in behavioral benefits. Behavioral testing showed that MPTP-injected mice exhibited motor deficits in the pole test and catalepsy test after 7 days, but arundic acid prevented the appearance of motor abnormalities in these tests. The MPTP-injected animals exhibited an 87% loss of tyrosine hydroxylase-containing dopaminergic neurons in the substantia nigra after 7 days, but the arundic acid-treated mice showed only a 56% reduction (p<0.01). GFAP-positive reactive astrocytes were accumulated in the striatum and substantia nigra 7 days after the MPTP injection, whereas arundic acid treatment induced an earlier appearance of reactive astrocytes by 3 days. The reactive astrocytes increased the production of S-100 protein, which is thought to promote neuronal damage, but arundic acid suppressed the expression of S-100. Thus, arundic acid protected dopaminergic neurons against MPTP neurotoxicity in mice and ameliorated neurological deficits. The results suggest that the neuroprotection is mediated through the modulation of astrocytic activation, including the inhibition of S-100 protein synthesis.
Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neurologic deficits.[Pubmed:12045671]
J Cereb Blood Flow Metab. 2002 Jun;22(6):723-34.
A novel agent, (R)-(-)-2-propyloctanoic acid (ONO-2506), has a unique property in that it modulates functions of activated cultured astrocytes, including pronounced inhibition of S-100beta synthesis. The present study examined whether administration of this agent would mitigate the delayed expansion of infarct volume and the neurologic deficits after permanent middle cerebral artery occlusion (pMCAO) in rats. Daily intravenous administration of ONO-2506 (10 mg/kg) abolished the delayed infarct expansion between 24 and 168 hours after pMCAO, whereas the acute infarct expansion until 24 hours was unaffected. The agent significantly reduced the expression of S-100beta and glial fibrillary acidic protein in the activated astrocytes and the number of terminal deoxynucleotidyl transferase-mediated 2;-deoxyuridine 5;-triphosphate-biotin nick end labeling-positive cells in the periinfarct area. The neurologic deficits were significantly improved, compared with the vehicle-treated groups, as early as 24 hours after the initial administration of ONO-2506. The agent had a wide therapeutic time window of 0 to 48 hours after pMCAO. These results indicate that because of the pharmacologic modulation of astrocytic activation induced by ONO-2506, symptoms can regress whereas delayed expansion of the lesion is arrested. Pharmacologic modulation of astrocytic activation may confer a novel therapeutic strategy against stroke.


