Corchoionoside CCAS# 185414-25-9 |
- Roseoside
Catalog No.:BCN5728
CAS No.:54835-70-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 185414-25-9 | SDF | Download SDF |
| PubChem ID | 10317980 | Appearance | Powder |
| Formula | C19H30O8 | M.Wt | 386.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
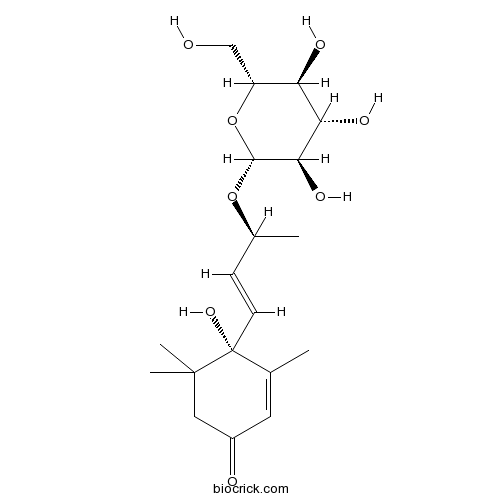
| Chemical Name | (4S)-4-hydroxy-3,5,5-trimethyl-4-[(E,3S)-3-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxybut-1-enyl]cyclohex-2-en-1-one | ||
| SMILES | CC1=CC(=O)CC(C1(C=CC(C)OC2C(C(C(C(O2)CO)O)O)O)O)(C)C | ||
| Standard InChIKey | SWYRVCGNMNAFEK-PUVRWCMWSA-N | ||
| Standard InChI | InChI=1S/C19H30O8/c1-10-7-12(21)8-18(3,4)19(10,25)6-5-11(2)26-17-16(24)15(23)14(22)13(9-20)27-17/h5-7,11,13-17,20,22-25H,8-9H2,1-4H3/b6-5+/t11-,13+,14+,15-,16+,17+,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Corchoionoside C has antioxidant activity, shows strong scavenging activities on DPPH radical. 2. Corchoionosides A, B, and C inhibit the histamine release from rat peritoneal exudate cells induced by antigen-antibody reaction. 3. Corchoionoside C shows weak antifungal activity. |
| Targets | Histamine Receptor | Antifection |

Corchoionoside C Dilution Calculator

Corchoionoside C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.588 mL | 12.94 mL | 25.8799 mL | 51.7598 mL | 64.6998 mL |
| 5 mM | 0.5176 mL | 2.588 mL | 5.176 mL | 10.352 mL | 12.94 mL |
| 10 mM | 0.2588 mL | 1.294 mL | 2.588 mL | 5.176 mL | 6.47 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5176 mL | 1.0352 mL | 1.294 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5176 mL | 0.647 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AF 12198
Catalog No.:BCC5812
CAS No.:185413-30-3
- O-Acetylethanolamine
Catalog No.:BCN1757
CAS No.:1854-30-4
- TCS 1105
Catalog No.:BCC6087
CAS No.:185391-33-7
- Fmoc-3-(2-Pyridyl)-Alanine
Catalog No.:BCC2568
CAS No.:185379-40-2
- Fmoc-3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2569
CAS No.:185379-39-9
- (R)-(+)-1,1'-Bi-2-naphthol
Catalog No.:BCC8393
CAS No.:18531-94-7
- GR 46611
Catalog No.:BCC5679
CAS No.:185259-85-2
- Rp-8-Br-PET-cGMPS
Catalog No.:BCC7538
CAS No.:185246-32-6
- Ceplignan
Catalog No.:BCN3626
CAS No.:185244-78-4
- Loganin
Catalog No.:BCN1153
CAS No.:18524-94-2
- Scabertopin
Catalog No.:BCN4685
CAS No.:185213-52-9
- Butabindide oxalate
Catalog No.:BCC7020
CAS No.:185213-03-0
- Timorsaponin C
Catalog No.:BCN2899
CAS No.:185432-00-2
- ONO 2506
Catalog No.:BCC7943
CAS No.:185517-21-9
- BMS 961
Catalog No.:BCC7680
CAS No.:185629-22-5
- Viroallosecurinine
Catalog No.:BCN6743
CAS No.:1857-30-3
- 2,3-Dihydroxypterodontic acid
Catalog No.:BCN1155
CAS No.:185821-32-3
- Pterodontic acid
Catalog No.:BCN1156
CAS No.:185845-89-0
- Echinulin
Catalog No.:BCN1157
CAS No.:1859-87-6
- H-Cys-OMe.HCl
Catalog No.:BCC2905
CAS No.:18598-63-5
- H-Ile-OMe.HCl
Catalog No.:BCC2964
CAS No.:18598-74-8
- AMD 3465 hexahydrobromide
Catalog No.:BCC5182
CAS No.:185991-07-5
- AMD 3465
Catalog No.:BCC5181
CAS No.:185991-24-6
- RS 79948 hydrochloride
Catalog No.:BCC6876
CAS No.:186002-54-0
Medicinal foodstuffs. V. Moroheiya. (1): Absolute stereostructures of corchoionosides A, B, and C, histamine release inhibitors from the leaves of Vietnamese Corchorus olitorius L. (Tiliaceae).[Pubmed:9085554]
Chem Pharm Bull (Tokyo). 1997 Mar;45(3):464-9.
Three new ionone glucosides named corchoionosides A, B, and C were isolated from the leaves of Corchorus olitorius, commonly called "moroheiya" in Japanese, together with seven known compounds, an ionone glucoside (6S,9R)-roseoside, a monoterpene glucoside betulalbuside A, two flavonol glucosides astragalin and isoquercitrin, two coumarin glucosides scopolin and cichoriine, and chlorogenic acid. The absolute stereostructures of corchoionosides A, B, and C were determined by chemical and physiochemical evidence, which included the result of application of a modified Mosher's method, the CD helicity rule, and chemical correlation with (6S,9R)-roseoside. Corchoionosides A and B and (6S,9R)-roseoside were found to inhibit the histamine release from rat peritoneal exudate cells induced by antigen-antibody reaction.
Isoswertisin flavones and other constituents from Peperomia obtusifolia.[Pubmed:21240754]
Nat Prod Res. 2011 Jan;25(1):1-7.
A phytochemical investigation of the leaves and stems of Peperomia obtusifolia (Piperaceae) yielded a new flavone C-diglycoside isoswertisin-4'-methyl-ether-2''alpha-L-rhamnoside (1), along with four known compounds: isoswertisin-2''alpha-L-rhamnoside (2), (+)-diayangambin (3), 2-episesalatin (4) and Corchoionoside C (5). The structures of the two flavone C-diglycosides (1, 2) were elucidated on the basis of 1D and 2D NMR spectroscopy and MS spectrometric data. These flavones were evaluated by bioautographic assay against Cladosporium cladosporioides and C. sphaerospermum and showed weak antifungal activity.
A new sulfated alpha-ionone glycoside from Sonchus erzincanicus Matthews.[Pubmed:20428066]
Molecules. 2010 Apr 12;15(4):2593-9.
Sonchus erzincanicus (Asteraceae) is an endemic species in Turkey, where six Sonchus species grow. In this study, a phytochemical study was performed on the aerial parts of the plant. The study describes the isolation and structure elucidation of five flavonoids and two a-ionone glycosides from S. erzincanicus. The compounds were isolated using several and repeated chromatographic techniques from ethyl acetate and aqueous phases that were partitioned from a methanol extract obtained from the plant. 5,7,3',4'-Tetrahydroxy-3-methoxyflavone (1) and quercetin 3-O-beta-D-glucoside (2) were isolated from the ethyl acetate phase, while Corchoionoside C 6'-O-sulfate (3), Corchoionoside C (4), luteolin 7-O-glucuronide (5) and luteolin 7-O-beta-D-glucoside (6), apigenin 7-O-glucuronide (7) were isolated from the aqueous phase. Corchoionoside C 6'-O-sulfate (3), isolated for the first time from a natural source, was a new compound. The structures of the compounds were elucidated by means of 1H-NMR, 13C-NMR, 2D-NMR (COSY, HMQC, HMBC) and ESI-MS.


