Butabindide oxalateCCK-inactivating serine protease inhibitor CAS# 185213-03-0 |
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 185213-03-0 | SDF | Download SDF |
| PubChem ID | 56972132 | Appearance | Powder |
| Formula | C19H27N3O6 | M.Wt | 393.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
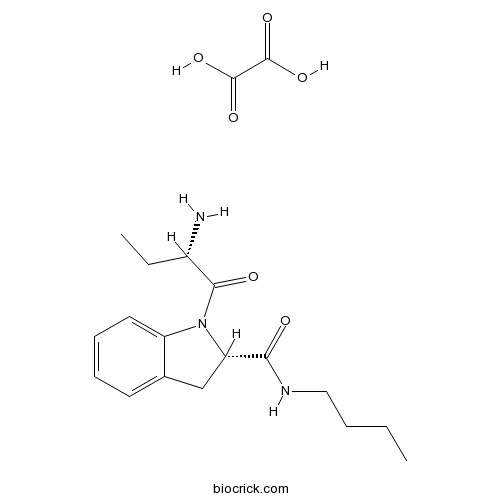
| Chemical Name | (2S)-1-[(2S)-2-aminobutanoyl]-N-butyl-2,3-dihydroindole-2-carboxamide;oxalic acid | ||
| SMILES | CCCCNC(=O)C1CC2=CC=CC=C2N1C(=O)C(CC)N.C(=O)(C(=O)O)O | ||
| Standard InChIKey | KKMJFDVOXSGHBF-SLHAJLBXSA-N | ||
| Standard InChI | InChI=1S/C17H25N3O2.C2H2O4/c1-3-5-10-19-16(21)15-11-12-8-6-7-9-14(12)20(15)17(22)13(18)4-2;3-1(4)2(5)6/h6-9,13,15H,3-5,10-11,18H2,1-2H3,(H,19,21);(H,3,4)(H,5,6)/t13-,15-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, reversible, selective and competitive inhibitor of a CCK-inactivating serine protease (tripeptidyl peptidase II) (Ki = 7 nM). Active in vivo (ID50 = 1.1 and 6.8 mg/kg i.v. for inhibition of liver and brain enzyme respectively). |

Butabindide oxalate Dilution Calculator

Butabindide oxalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5417 mL | 12.7084 mL | 25.4168 mL | 50.8337 mL | 63.5421 mL |
| 5 mM | 0.5083 mL | 2.5417 mL | 5.0834 mL | 10.1667 mL | 12.7084 mL |
| 10 mM | 0.2542 mL | 1.2708 mL | 2.5417 mL | 5.0834 mL | 6.3542 mL |
| 50 mM | 0.0508 mL | 0.2542 mL | 0.5083 mL | 1.0167 mL | 1.2708 mL |
| 100 mM | 0.0254 mL | 0.1271 mL | 0.2542 mL | 0.5083 mL | 0.6354 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chrysin 6-C-glucoside 8-C-arabinoside
Catalog No.:BCN1516
CAS No.:185145-34-0
- Chrysin 6-C-arabinoside 8-C-glucoside
Catalog No.:BCN1517
CAS No.:185145-33-9
- Ethyl 1,2,5,6-tetrahydropyridine-3-carboxylate
Catalog No.:BCC8299
CAS No.:18513-76-3
- Decoquinate
Catalog No.:BCC4654
CAS No.:18507-89-6
- R 715
Catalog No.:BCC6014
CAS No.:185052-09-9
- Liquidambaric lactone
Catalog No.:BCN2301
CAS No.:185051-75-6
- Brevifolincarboxylic acid
Catalog No.:BCN3884
CAS No.:18490-95-4
- 5-Benzyl-1H-tetrazole
Catalog No.:BCC8741
CAS No.:18489-25-3
- NNC 05-2090 hydrochloride
Catalog No.:BCC7472
CAS No.:184845-18-9
- 1,3,6-Tri-O-galloylglucose
Catalog No.:BCN8227
CAS No.:18483-17-5
- Chlorhexidine digluconate
Catalog No.:BCC5264
CAS No.:18472-51-0
- Delta 5-avenasterol
Catalog No.:BCN3211
CAS No.:18472-36-1
- Scabertopin
Catalog No.:BCN4685
CAS No.:185213-52-9
- Loganin
Catalog No.:BCN1153
CAS No.:18524-94-2
- Ceplignan
Catalog No.:BCN3626
CAS No.:185244-78-4
- Rp-8-Br-PET-cGMPS
Catalog No.:BCC7538
CAS No.:185246-32-6
- GR 46611
Catalog No.:BCC5679
CAS No.:185259-85-2
- (R)-(+)-1,1'-Bi-2-naphthol
Catalog No.:BCC8393
CAS No.:18531-94-7
- Fmoc-3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2569
CAS No.:185379-39-9
- Fmoc-3-(2-Pyridyl)-Alanine
Catalog No.:BCC2568
CAS No.:185379-40-2
- TCS 1105
Catalog No.:BCC6087
CAS No.:185391-33-7
- O-Acetylethanolamine
Catalog No.:BCN1757
CAS No.:1854-30-4
- AF 12198
Catalog No.:BCC5812
CAS No.:185413-30-3
- Corchoionoside C
Catalog No.:BCN1154
CAS No.:185414-25-9
Inhibitors of tripeptidyl peptidase II. 2. Generation of the first novel lead inhibitor of cholecystokinin-8-inactivating peptidase: a strategy for the design of peptidase inhibitors.[Pubmed:10691692]
J Med Chem. 2000 Feb 24;43(4):664-74.
The cholecystokinin-8 (CCK-8)-inactivating peptidase is a serine peptidase which has been shown to be a membrane-bound isoform of tripeptidyl peptidase II (EC 3.4.14.10). It cleaves the neurotransmitter CCK-8 sulfate at the Met-Gly bond to give Asp-Tyr(SO(3)H)-Met-OH + Gly-Trp-Met-Asp-Phe-NH(2). In seeking a reversible inhibitor of this peptidase, the enzymatic binding subsites were characterized using a fluorimetric assay based on the hydrolysis of the artificial substrate Ala-Ala-Phe-amidomethylcoumarin. A series of di- and tripeptides having various alkyl or aryl side chains was studied to determine the accessible volume for binding and to probe the potential for hydrophobic interactions. From this initial study the tripeptides Ile-Pro-Ile-OH (K(i) = 1 microM) and Ala-Pro-Ala-OH (K(i) = 3 microM) and dipeptide amide Val-Nvl-NHBu (K(i) = 3 microM) emerged as leads. Comparison of these structures led to the synthesis of Val-Pro-NHBu (K(i) = 0.57 microM) which served for later optimization in the design of butabindide, a potent reversible competitive and selective inhibitor of the CCK-8-inactivating peptidase. The strategy for this work is explicitly described since it illustrates a possible general approach for peptidase inhibitor design.
Characterization and cloning of tripeptidyl peptidase II from the fruit fly, Drosophila melanogaster.[Pubmed:9668104]
J Biol Chem. 1998 Jul 24;273(30):19173-82.
We describe the characterization, cloning, and genetic analysis of tripeptidyl peptidase II (TPP II) from Drosophila melanogaster. Mammalian TPP II removes N-terminal tripeptides, has wide distribution, and has been identified as the cholecystokinin-degrading peptidase in rat brain. Size exclusion and ion exchange chromatography produced a 70-fold purification of dTPP II activity from Drosophila tissue extracts. The substrate specificity and the inhibitor sensitivity of dTPP II is comparable to that of the human enzyme. In particular, dTPP II is sensitive to butabindide, a specific inhibitor of the rat cholecystokinin-inactivating activity. We isolated a 4309-base pair dTPP II cDNA which predicts a 1354-amino acid protein. The deduced human and Drosophila TPP II proteins display 38% overall identity. The catalytic triad, its spacing, and the sequences that surround it are highly conserved; the C-terminal end of dTPP II contains a 100-amino acid insert not found in the mammalian proteins. Recombinant dTPP II displays the predicted activity following expression in HEK cells. TPP II maps to cytological position 49F4-7; animals deficient for this interval show reduced TPP II activity.
Characterization and inhibition of a cholecystokinin-inactivating serine peptidase.[Pubmed:8602240]
Nature. 1996 Apr 4;380(6573):403-9.
A cholecystokinin (CCK)-inactivating peptidase was purified and identified as a membrane-bound isoform of tripeptidyl peptidase II (EC 3.4.14.10), a cytosolic subtilisin-like peptidase of previously unknown functions. The peptidase was found in neurons responding to cholecystokinin, as well as in non-neuronal cells. Butabindide, a potent and specific inhibitor, was designed and shown to protect endogenous cholecystokinin from inactivation and to display pro-satiating effects mediated by the CCKA receptor.


