R 715Potent and selective B1 antagonist CAS# 185052-09-9 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 185052-09-9 | SDF | Download SDF |
| PubChem ID | 5311397 | Appearance | Powder |
| Formula | C57H81N13O12 | M.Wt | 1140.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mg/ml in water | ||
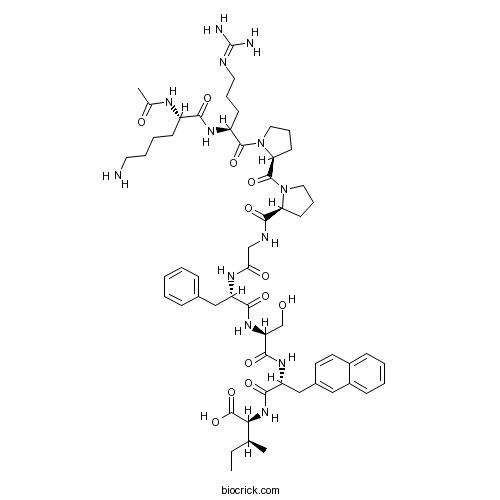
| Sequence | KRPPGFSXI (Modifications: Lys-1 = N-terminal Ac, X = DβNal) | ||
| Chemical Name | (2S,3S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-1-[(2S)-1-[(2S)-2-[[(2S)-2-acetamido-6-aminohexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]pyrrolidine-2-carbonyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-hydroxypropanoyl]amino]-3-naphthalen-2-ylpropanoyl]amino]-3-methylpentanoic acid | ||
| SMILES | CCC(C)C(C(=O)O)NC(=O)C(CC1=CC2=CC=CC=C2C=C1)NC(=O)C(CO)NC(=O)C(CC3=CC=CC=C3)NC(=O)CNC(=O)C4CCCN4C(=O)C5CCCN5C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C | ||
| Standard InChIKey | DOSXOGUJJBDRGQ-VUBDHFCFSA-N | ||
| Standard InChI | InChI=1S/C57H81N13O12/c1-4-34(2)48(56(81)82)68-51(76)43(31-37-23-24-38-17-8-9-18-39(38)29-37)66-52(77)44(33-71)67-50(75)42(30-36-15-6-5-7-16-36)64-47(73)32-62-53(78)45-21-13-27-69(45)55(80)46-22-14-28-70(46)54(79)41(20-12-26-61-57(59)60)65-49(74)40(63-35(3)72)19-10-11-25-58/h5-9,15-18,23-24,29,34,40-46,48,71H,4,10-14,19-22,25-28,30-33,58H2,1-3H3,(H,62,78)(H,63,72)(H,64,73)(H,65,74)(H,66,77)(H,67,75)(H,68,76)(H,81,82)(H4,59,60,61)/t34-,40-,41-,42-,43+,44-,45-,46-,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective bradykinin B1 receptor antagonist (pA2 = 8.49). Displays no activity at B2 receptors. Reduces mechanical hypernociception in a mouse model of neuropathic pain. Metabolically stable. |

R 715 Dilution Calculator

R 715 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Liquidambaric lactone
Catalog No.:BCN2301
CAS No.:185051-75-6
- Brevifolincarboxylic acid
Catalog No.:BCN3884
CAS No.:18490-95-4
- 5-Benzyl-1H-tetrazole
Catalog No.:BCC8741
CAS No.:18489-25-3
- NNC 05-2090 hydrochloride
Catalog No.:BCC7472
CAS No.:184845-18-9
- 1,3,6-Tri-O-galloylglucose
Catalog No.:BCN8227
CAS No.:18483-17-5
- Chlorhexidine digluconate
Catalog No.:BCC5264
CAS No.:18472-51-0
- Delta 5-avenasterol
Catalog No.:BCN3211
CAS No.:18472-36-1
- Nortropacocaine
Catalog No.:BCN1889
CAS No.:18470-33-2
- Sodium houttuyfonate
Catalog No.:BCN2978
CAS No.:1847-58-1
- NAD 299 hydrochloride
Catalog No.:BCC6003
CAS No.:184674-99-5
- Pelargonidin-3-O-glucoside chloride
Catalog No.:BCN3113
CAS No.:18466-51-8
- 2-C-Methyl-D-erythrono-1,4-lactone
Catalog No.:BCN4769
CAS No.:18465-71-9
- Decoquinate
Catalog No.:BCC4654
CAS No.:18507-89-6
- Ethyl 1,2,5,6-tetrahydropyridine-3-carboxylate
Catalog No.:BCC8299
CAS No.:18513-76-3
- Chrysin 6-C-arabinoside 8-C-glucoside
Catalog No.:BCN1517
CAS No.:185145-33-9
- Chrysin 6-C-glucoside 8-C-arabinoside
Catalog No.:BCN1516
CAS No.:185145-34-0
- Butabindide oxalate
Catalog No.:BCC7020
CAS No.:185213-03-0
- Scabertopin
Catalog No.:BCN4685
CAS No.:185213-52-9
- Loganin
Catalog No.:BCN1153
CAS No.:18524-94-2
- Ceplignan
Catalog No.:BCN3626
CAS No.:185244-78-4
- Rp-8-Br-PET-cGMPS
Catalog No.:BCC7538
CAS No.:185246-32-6
- GR 46611
Catalog No.:BCC5679
CAS No.:185259-85-2
- (R)-(+)-1,1'-Bi-2-naphthol
Catalog No.:BCC8393
CAS No.:18531-94-7
- Fmoc-3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2569
CAS No.:185379-39-9
Beneficial effect of chronic treatment with the selective bradykinin B1 receptor antagonists, R-715 and R-954, in attenuating streptozotocin-diabetic thermal hyperalgesia in mice.[Pubmed:14612183]
Peptides. 2003 Aug;24(8):1131-9.
Kinins are important mediators of cardiovascular homeostasis, inflammation and nociception. Bradykinin (BK) B(1) receptors (BKB1-R) are over-expressed in pathological conditions including diabetes, and were reported to play a role in hyperglycemia, renal abnormalities, and altered vascular permeability associated with type 1 diabetes. Recent studies from our laboratory demonstrated that BKB1-R are implicated in streptozotocin (STZ)-diabetes-mediated hyperalgesia, since acute administration of the selective BKB1-R antagonists significantly and dose-dependently inhibited such hyperalgesic activity. In the present study, we examined the effect of chronic treatment of STZ-diabetic mice with the selective BKB1-R agonist desArg9bradykinin (DBK) and two specific antagonists R-715 and R-954, on diabetic hyperalgesia. Diabetes was induced in male CD-1 mice by injecting a single high dose of STZ (200mg/kg, i.p.) and nociception was assessed using the hot plate, plantar stimulation, tail immersion and tail flick tests. Drugs were injected i.p. twice daily for 7 days, starting 4 days after STZ. We showed that chronically administered R-715 (400 micrograms/kg) and R-954 (200 micrograms/kg), significantly attenuated the hyperalgesic effect developed in STZ-diabetic mice as measured by the four thermal nociceptive tests. Further, chronic treatment with DBK (400 micrograms/kg) produced a marked potentiation of the hyperalgesic activity, an effect that was reversed by both R-715 and R-954. The results from this chronic study confirm a pivotal role of the BKB1-R in the development of STZ-diabetic hyperalgesia and suggest a novel approach to the treatment of this short-term diabetic complication using BKB1-R antagonists.
Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors.[Pubmed:18311190]
Br J Pharmacol. 2008 May;154(1):136-43.
BACKGROUND AND PURPOSE: We investigated whether or not kinin receptors play a role in diabetic blood-retinal barrier breakdown, which is a leading cause of vision loss. EXPERIMENTAL APPROACH: Blood-retinal barrier breakdown was quantified using Evans blue, and expression of kinin B(1) receptor mRNA was measured using quantitative reverse transcrition-PCR. Diabetic rats (streptozotocin (STZ), 65 mg kg(-1)) received a single intraocular injection of bradykinin (BK) or des-Arg(9)-BK, alone, or in combination with antagonists for B(1) (des-Arg(10)-Hoe140, R-715) and/or B(2) (Hoe140) receptors, given intraocularly or intravenously (i.v.). KEY RESULTS: In control rats, BK (0.1-10 nmol) dose-dependently increased plasma extravasation, which was inhibited by Hoe140 (0.2 nmol), whereas des-Arg(9)-BK (0.1 and 1 nmol) was without effect. B(1) receptor mRNA was markedly increased in retinas of diabetic rats, and this was prevented by N-acetyl-L-cysteine (1 g kg(-1) day(-1) for 7 days). Plasma extravasation in retinas of STZ-diabetic rats was higher than in controls and enhanced by des-Arg(9)-BK. Response to des-Arg(9)-BK was inhibited by intraocular or i.v. injection of B(1) receptor antagonists. Diabetes-induced plasma extravasation was inhibited only by a combination of des-Arg(10)-Hoe140 and Hoe 140 (100 nmol kg(-1), i.v. 15 min earlier) or by R-715 (1 micromol kg(-1), i.v.) injected daily for 7 days. CONCLUSIONS AND IMPLICATIONS: Kinin B(1) receptors are upregulated in retinas of STZ-diabetic rats through a mechanism involving oxidative stress. Both kinin B(1) and B(2) receptors contribute to increased plasma extravasation in diabetic retinopathy. Chronic inhibition of both kinin receptors, possibly with antioxidant adjuvants, may be a novel therapeutic strategy for diabetic retinopathy.
Neuropathic pain-like behavior after brachial plexus avulsion in mice: the relevance of kinin B1 and B2 receptors.[Pubmed:18337416]
J Neurosci. 2008 Mar 12;28(11):2856-63.
The relevance of kinin B(1) (B(1)R) and B(2) (B(2)R) receptors in the brachial plexus avulsion (BPA) model was evaluated in mice, by means of genetic and pharmacological tools. BPA-induced hypernociception was absent in B(1)R, but not in B(2)R, knock-out mice. Local or intraperitoneal administration of the B(2)R antagonist Hoe 140 failed to affect BPA-induced mechanical hypernociception. Interestingly, local or intraperitoneal treatment with B(1)R antagonists, R-715 or SSR240612, dosed at the time of surgery, significantly reduced BPA-evoked mechanical hypernociception. Intrathecal or intracerebroventricular administration of these antagonists, at the surgery moment, did not prevent the hypernociception. Both antagonists, dosed by intraperitoneal or intrathecal routes (but not intracerebroventricularly) 4 d after the surgery, significantly inhibited the mechanical hypernociception. At 30 d after the BPA, only the intracerebroventricular treatment effectively reduced the hypernociception. A marked increase in B(1)R mRNA was observed in the hypothalamus, hippocampus, thalamus, and cortex at 4 d after BPA and only in the hypothalamus and cortex at 30 d. In the spinal cord, a slight increase in B(1)R mRNA expression was observed as early as at 2 d. Finally, an enhancement of B(1)R protein expression was found in all the analyzed brain structures at 4 and 30 d after the BPA, whereas in the spinal cord, this parameter was augmented only at 4 d. The data provide new evidence on the role of peripheral and central kinin B(1)R in the BPA model of neuropathic pain. Selective B(1)R antagonists might well represent valuable tools for the management of neuropathic pain.
Structure-activity studies of B1 receptor-related peptides. Antagonists.[Pubmed:8901831]
Hypertension. 1996 Nov;28(5):833-9.
We tested several peptides related to des-Arg9-bradykinin as stimulants or inhibitors of B1 (rabbit aorta, human umbilical vein) and B2 (rabbit jugular vein, guinea pig ileum, human umbilical vein) receptors. We also incubated the compounds with purified angiotensin-converting enzyme from rabbit lung to test their resistance to degradation. We evaluated apparent affinities (in terms of the affinity constant pA2) of compounds and their potential residual agonistic activities (alpha E). Bradykinin and des-Arg9-bradykinin were used as agonists for the B2 and B1 receptors, respectively. Degradation of peptides by the angiotensin-converting enzyme was prevented in the presence of a D-residue in position 7 of des-Arg9-bradykinin. Replacement of Pro7 with D-Tic combined with Leu, Ile, Ala, or D-Tic in position 8 led to weak B1 receptor antagonists, some of which had strong residual agonistic activities on the B2 receptor preparations. The use of D-beta Nal in position 7, combined with Ile in position 8 and AcLys at the N-terminal (eg, AcLys[D-beta Nal7, Ile8]des-Arg9-bradykinin) gave the most active B1 receptor antagonist (pA2 of 8.5 on rabbit aorta and human umbilical vein), which is also partially resistant to enzymatic degradation. Extension of the N-terminal end by Sar-Tyr-epsilon Ahx (used for labeling purposes) and even cold-labeling of Tyr with iodine were compatible with high, selective, and specific antagonism of the B1 receptors. We compared some compounds with some already known B1 receptor antagonists to underline the novelty of new peptidic compounds.


