TCS 1105CAS# 185391-33-7 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 185391-33-7 | SDF | Download SDF |
| PubChem ID | 2986030 | Appearance | Powder |
| Formula | C17H13FN2O2 | M.Wt | 296.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in ethanol | ||
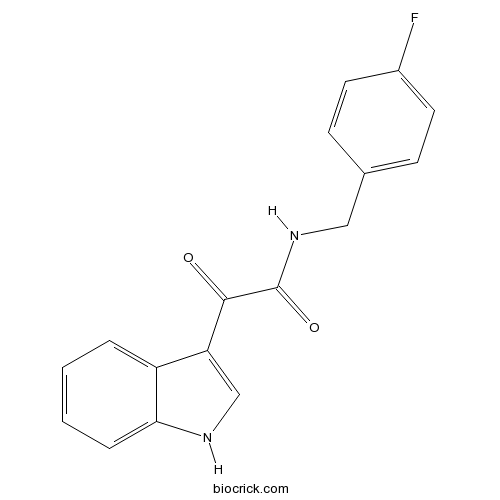
| Chemical Name | N-[(4-fluorophenyl)methyl]-2-(1H-indol-3-yl)-2-oxoacetamide | ||
| SMILES | C1=CC=C2C(=C1)C(=CN2)C(=O)C(=O)NCC3=CC=C(C=C3)F | ||
| Standard InChIKey | VWCCHJFFYCGXFL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H13FN2O2/c18-12-7-5-11(6-8-12)9-20-17(22)16(21)14-10-19-15-4-2-1-3-13(14)15/h1-8,10,19H,9H2,(H,20,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GABAA benzodiazepine receptor (BZR) ligand. Acts as an agonist at α2 and antagonist at α1 benzodiazepine receptors (Ki values are 118 and 245 nM respectively). Anxiolytic agent lacking sedative activity. |

TCS 1105 Dilution Calculator

TCS 1105 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.375 mL | 16.8748 mL | 33.7496 mL | 67.4992 mL | 84.3739 mL |
| 5 mM | 0.675 mL | 3.375 mL | 6.7499 mL | 13.4998 mL | 16.8748 mL |
| 10 mM | 0.3375 mL | 1.6875 mL | 3.375 mL | 6.7499 mL | 8.4374 mL |
| 50 mM | 0.0675 mL | 0.3375 mL | 0.675 mL | 1.35 mL | 1.6875 mL |
| 100 mM | 0.0337 mL | 0.1687 mL | 0.3375 mL | 0.675 mL | 0.8437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-3-(2-Pyridyl)-Alanine
Catalog No.:BCC2568
CAS No.:185379-40-2
- Fmoc-3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2569
CAS No.:185379-39-9
- (R)-(+)-1,1'-Bi-2-naphthol
Catalog No.:BCC8393
CAS No.:18531-94-7
- GR 46611
Catalog No.:BCC5679
CAS No.:185259-85-2
- Rp-8-Br-PET-cGMPS
Catalog No.:BCC7538
CAS No.:185246-32-6
- Ceplignan
Catalog No.:BCN3626
CAS No.:185244-78-4
- Loganin
Catalog No.:BCN1153
CAS No.:18524-94-2
- Scabertopin
Catalog No.:BCN4685
CAS No.:185213-52-9
- Butabindide oxalate
Catalog No.:BCC7020
CAS No.:185213-03-0
- Chrysin 6-C-glucoside 8-C-arabinoside
Catalog No.:BCN1516
CAS No.:185145-34-0
- Chrysin 6-C-arabinoside 8-C-glucoside
Catalog No.:BCN1517
CAS No.:185145-33-9
- Ethyl 1,2,5,6-tetrahydropyridine-3-carboxylate
Catalog No.:BCC8299
CAS No.:18513-76-3
- O-Acetylethanolamine
Catalog No.:BCN1757
CAS No.:1854-30-4
- AF 12198
Catalog No.:BCC5812
CAS No.:185413-30-3
- Corchoionoside C
Catalog No.:BCN1154
CAS No.:185414-25-9
- Timorsaponin C
Catalog No.:BCN2899
CAS No.:185432-00-2
- ONO 2506
Catalog No.:BCC7943
CAS No.:185517-21-9
- BMS 961
Catalog No.:BCC7680
CAS No.:185629-22-5
- Viroallosecurinine
Catalog No.:BCN6743
CAS No.:1857-30-3
- 2,3-Dihydroxypterodontic acid
Catalog No.:BCN1155
CAS No.:185821-32-3
- Pterodontic acid
Catalog No.:BCN1156
CAS No.:185845-89-0
- Echinulin
Catalog No.:BCN1157
CAS No.:1859-87-6
- H-Cys-OMe.HCl
Catalog No.:BCC2905
CAS No.:18598-63-5
- H-Ile-OMe.HCl
Catalog No.:BCC2964
CAS No.:18598-74-8
Identification of anxiolytic/nonsedative agents among indol-3-ylglyoxylamides acting as functionally selective agonists at the gamma-aminobutyric acid-A (GABAA) alpha2 benzodiazepine receptor.[Pubmed:19469479]
J Med Chem. 2009 Jun 25;52(12):3723-34.
Anxioselective agents may be identified among compounds binding selectively to the alpha(2)beta(x)gamma(2) subtype of the gamma-aminobutyric acid-A (GABA(A))/central benzodiazepine receptor (BzR) complex and behaving as agonists or among compounds binding with comparable potency to various BzR subtypes but eliciting agonism only at the alpha(2)beta(x)gamma(2) receptor. Because of subtle steric differences among BzR subtypes, the latter approach has proved much more successful. A biological screening within the class of indol-3-ylglyoxylamides 1-3 allowed us to identify compounds 1c and 2b as potential anxiolytic/nonsedative agents showing alpha(2) selective efficacy in vitro and anxioselective effects in vivo. According to molecular modeling studies, and consistently with SARs accumulated in the past decade, 5-NO(2)- and 5-H-indole derivatives would preferentially bind to BzR by placing the indole ring in the L(Di) and the L(2) receptor binding sites, respectively.


