Desvenlafaxine SuccinateCAS# 386750-22-7 |
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Desmopressin Acetate
Catalog No.:BCC1526
CAS No.:62288-83-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 386750-22-7 | SDF | Download SDF |
| PubChem ID | 6918664 | Appearance | Powder |
| Formula | C20H33NO7 | M.Wt | 399.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | O-Desmethylvenlafaxine succinate hydrate | ||
| Solubility | DMSO : ≥ 33 mg/mL (82.61 mM) *"≥" means soluble, but saturation unknown. | ||
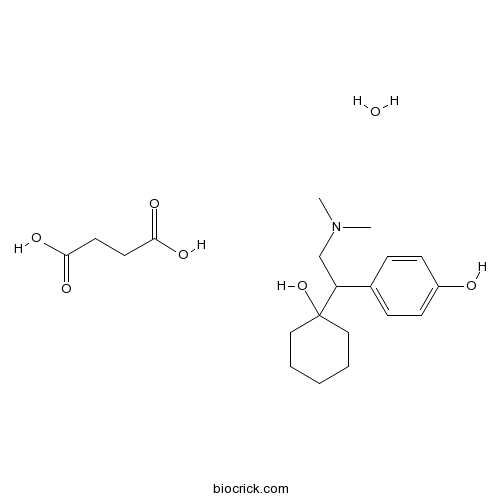
| Chemical Name | butanedioic acid;4-[2-(dimethylamino)-1-(1-hydroxycyclohexyl)ethyl]phenol;hydrate | ||
| SMILES | CN(C)CC(C1=CC=C(C=C1)O)C2(CCCCC2)O.C(CC(=O)O)C(=O)O.O | ||
| Standard InChIKey | PWPDEXVGKDEKTE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H25NO2.C4H6O4.H2O/c1-17(2)12-15(13-6-8-14(18)9-7-13)16(19)10-4-3-5-11-16;5-3(6)1-2-4(7)8;/h6-9,15,18-19H,3-5,10-12H2,1-2H3;1-2H2,(H,5,6)(H,7,8);1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Desvenlafaxine succinate hydrate is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI). |

Desvenlafaxine Succinate Dilution Calculator

Desvenlafaxine Succinate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5031 mL | 12.5156 mL | 25.0313 mL | 50.0626 mL | 62.5782 mL |
| 5 mM | 0.5006 mL | 2.5031 mL | 5.0063 mL | 10.0125 mL | 12.5156 mL |
| 10 mM | 0.2503 mL | 1.2516 mL | 2.5031 mL | 5.0063 mL | 6.2578 mL |
| 50 mM | 0.0501 mL | 0.2503 mL | 0.5006 mL | 1.0013 mL | 1.2516 mL |
| 100 mM | 0.025 mL | 0.1252 mL | 0.2503 mL | 0.5006 mL | 0.6258 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Desvenlafaxine Succinate is a new serotonin (5-HT) transporter and norepinephrine (NE) transporter reuptake inhibitor with Ki of 40.2 nM and 558.4 nM respectively.
- Neodiosmin
Catalog No.:BCN8337
CAS No.:38665-01-9
- Triptonide
Catalog No.:BCN5924
CAS No.:38647-11-9
- Tripdiolide
Catalog No.:BCN5985
CAS No.:38647-10-8
- Benzoylpaeoniflorin
Catalog No.:BCN6293
CAS No.:38642-49-8
- Lasiodonin
Catalog No.:BCN7156
CAS No.:38602-52-7
- Chloroprocaine HCl
Catalog No.:BCC5556
CAS No.:3858-89-7
- Prostaglandin F2α
Catalog No.:BCC7889
CAS No.:38562-01-5
- Oxotremorine M
Catalog No.:BCC6920
CAS No.:3854-04-4
- Daphnicyclidin H
Catalog No.:BCN7080
CAS No.:385384-29-2
- Daphnicyclidin F
Catalog No.:BCN6400
CAS No.:385384-26-9
- Daphnicyclidin D
Catalog No.:BCN7081
CAS No.:385384-24-7
- Tarafenacin
Catalog No.:BCC4147
CAS No.:385367-47-5
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Groenlandicine
Catalog No.:BCN8189
CAS No.:38691-95-1
- Preskimmianine
Catalog No.:BCN6667
CAS No.:38695-41-9
- Seneciphylline N-oxide
Catalog No.:BCN5439
CAS No.:38710-26-8
- 3-Epibetulinic acid
Catalog No.:BCN8531
CAS No.:38736-77-5
- Triptolide
Catalog No.:BCN5984
CAS No.:38748-32-2
- Worenine
Catalog No.:BCN2557
CAS No.:38763-29-0
- Swazine
Catalog No.:BCN2143
CAS No.:38763-74-5
- Cucurbitacin D
Catalog No.:BCN2355
CAS No.:3877-86-9
- Tetrahydroisocucurbitacin I
Catalog No.:BCN7874
CAS No.:3877-89-2
- Muristerone A
Catalog No.:BCC2397
CAS No.:38778-30-2
- Tectoruside
Catalog No.:BCN8262
CAS No.:38784-73-5
Efficacy of desvenlafaxine succinate for menopausal hot flashes.[Pubmed:25252697]
Expert Opin Pharmacother. 2014 Nov;15(16):2407-18.
INTRODUCTION: The concern for the development of breast cancer, stroke, cardiovascular disease and deep venous thrombosis with the use of hormonal therapy has led to the development of alternative nonhormonal forms of therapy like desvenlafaxine for the management of hot flashes. AREAS COVERED: This review is based upon a PubMed search and clinical trials. The pharmacokinetics and pharmacodynamics of desvenlafaxine are reviewed. This review outlines the effects of desvenlafaxine in management of severity and frequency of vasomotor symptoms, sleep quality and quality of life in postmenopausal women. The potential adverse effects of desvenlafaxine are summarized. EXPERT OPINION: Based on the evidence from randomized clinical trials, desvenlafaxine is an alternate viable option for reducing the frequency and severity of hot flashes when other treatments fail. In clinical trials, it has been shown that desvenlafaxine reduced the frequency of hot flashes by 55 - 69%. In the trials so far it appears to have good safety and tolerability profile when the drug is initiated in titrating doses. The optimum dose is 100 mg/day and is to be started at 50 mg/day for 3 days and titrated to 100 mg/day. The most common adverse events reported were nausea, dry mouth, fatigue, constipation, diarrhea and somnolence.
[Determination of succinic acid in desvenlafaxine succinate by high performance ion-exclusion chromatography and high performance ion-exchange chromatography].[Pubmed:27382725]
Se Pu. 2016 Feb;34(2):189-93.
New methods were developed for the determination of succinic acid in Desvenlafaxine Succinate (DVS) by high performance ion-exclusion chromatography (HPIEC) and high performance ion-exchange chromatography (HPIC). HPIEC and HPIC methods were used separately to determinate the succinic acid in DVS. With HPIEC, the sample was diluted with 2. 50 x 10(-3) mol/L sulfuric acid solution and filtrated by 0. 22 microm polyether sulfone filter membrane, and then analyzed by HPIEC directly without any further pretreatment. The analytical column was Phenomenex Rezex ROA-organic Acid H+(8%) (300 mmx7. 8 mm). The mobile phase was 2. 50x10(-3) mol/L sulfuric acid solution at the flow rate of 0. 5 mL/min. The column temperature was set at 40 degrees C, and the detection wavelength was 210 nm. The injection volume was 10 KL. The assay was quantified by external standard method. With HPIC, the sample was diluted with ultrapure water and filtrated by 0. 22 microm polyether sulfone filter membrane, and then analyzed by HPIC directly without any further pretreatment. The analytical column was Dionex IonPac AS11-HC (250 mm x 4 mm) with a guard column IonPacAG11-HC (50 mm x 4 mm). Isocratic KOH elute generator was used at the flow rate of 1. 0 mL/min. The detection was performed by a Dionex suppressed (DIONEX AERS 500 4-mm) conductivity detector. The injection volume was 10 microL. The content computation was performed with peak area external reference method. The results of HPIEC method for succinic acid were 28. 8%, 28. 9% and 28. 9%, while the results of HPIEC method were 28. 2%, 28. 6% and 28. 6%. The results of HPIEC and HPIC methods were not significantly different. The two methods can both be used to determine the contents of succinic acid in DVS. The surveillance analytical method should be chosen according to the situation.
Desvenlafaxine succinate ameliorates visceral hypersensitivity but delays solid gastric emptying in rats.[Pubmed:23764892]
Am J Physiol Gastrointest Liver Physiol. 2013 Aug 15;305(4):G333-9.
Desvenlafaxine Succinate (DVS) is a novel serotonin and norepinephrine reuptake inhibitor. The aim of this study was to investigate the effects of DVS on visceral hypersensitivity and solid gastric emptying in a rodent model of gastric hyperalgesia. Twenty-eight gastric hyperalgesia rats and 20 control rats were used. Visceral sensitivity during gastric distention (GD) was assessed by recording of electromyogram (EMG) at pressures of 20, 40, 60, and 80 mmHg. DVS with doses of 1, 10, and 30 mg/kg were administrated by gavage, 5-HT1A antagonist (WAY-100635, 0.3 mg/kg) was given subcutaneously, and 5-HT2A antagonist (ketanserin, 1 mg/kg) was given intraperitoneally. The level of norepinephrine in plasma was measured by enzyme-linked immunosorbent assay. We found that 1) visceral hypersensitivity induced by acetic acid was validated. 2) DVS dose-dependently reduced visceral hypersensitivity in the gastric hypersensitivity rats. The EMG (% of baseline value without GD) during GD at 60 and 80 mmHg with DVS at a dose of 30 mg/kg were 119.4 +/- 2.3% (vs. saline 150.9 +/- 2.7%, P < 0.001) and 128.2 +/- 3.2% (vs. saline 171.1 +/- 2.4%, P < 0.001). Similar findings were observed at a dose of 10 mg/kg. DVS at a dose of 1 mg/kg reduced visceral hypersensitivity only during GD at 60 mmHg. 3) Neither WAY-100635 nor ketanserin blocked the effect of DVS on visceral sensitivity. 4) DVS at 30 mg/kg significantly increased plasma NE level (P = 0.012 vs. saline). 5) DVS at 30 mg/kg significantly delayed solid gastric emptying (P < 0.05 vs. saline). We conclude that DVS reduces visceral sensitivity in a rodent model of visceral hypersensitivity and delays solid gastric emptying. Caution should be made when DVS is used for treating patients.
A Multi-layered Particulate System for Desvenlafaxine Succinate Oral Customized Release.[Pubmed:27211103]
Curr Drug Deliv. 2017;14(3):416-425.
BACKGROUND: With its reported side effects Desvenlafaxine Succinate (DSV) is a good candidate to prepare prolonged release system. Such prolonged release could decrease the rapid DSV absorption after oral administration and reduce its exaggerated side effects. METHODS: A prolonged release Desvenlafaxine Succinate (DSV) multilayered system was prepared by ionotropic gelation using sodium alginate (SA) and calcium chloride as a cross-linker. DSV was incorporated simultaneously during the gelation stage and the formed beads were evaluated for shape and particle size. Thirteen formulation variables including pH, DSV: polymer ratio, cross-linker concentration and curing time were optimized for optimal drug entrapment. The optimized formula was evaluated ex vivo using the everted sac technique to predict DSV absorption through intestinal mucosal cells, follow the permeation and calculate its apparent permeability coefficient. RESULTS: The optimum formulation variables were: pH (8-9), DSV: SA ratio (2:1), cross-linker concentration (5%w/v) and 30 min curing time. Multilayered beads coating using chitosan and SA was compared with uncoated beads or the innovator for DSV release. Coating of the beads greatly retarded DSV release with a release profile similar to that of the innovator. An optimized formula (T13) coated with 0.04% w/v of each of chitosan and SA was selected. The developed system gave rise to a prolonged release pattern with high similarity factor with the innovator. CONCLUSION: The results of the current work can be applied to prepare controlled release systems of similar drugs that have intense side effects associated with their initial burst after oral administration.


