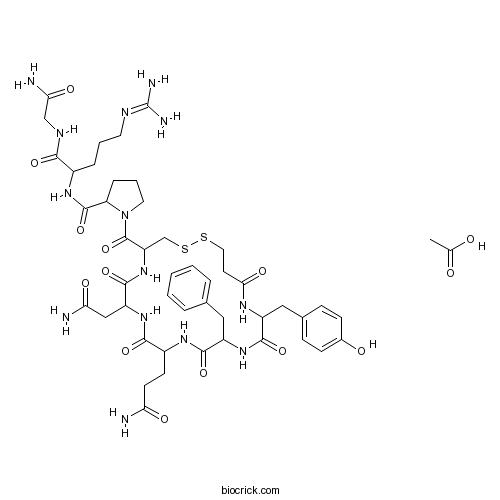
Desmopressin AcetateSynthetic analogue of arginine vasopressin CAS# 62288-83-9 |
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62288-83-9 | SDF | Download SDF |
| PubChem ID | 64758 | Appearance | Powder |
| Formula | C48H68N14O14S2 | M.Wt | 1129.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DDAVP | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | acetic acid;N-[1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]-1-[7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamide | ||
| SMILES | CC(=O)O.C1CC(N(C1)C(=O)C2CSSCCC(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N2)CC(=O)N)CCC(=O)N)CC3=CC=CC=C3)CC4=CC=C(C=C4)O)C(=O)NC(CCCN=C(N)N)C(=O)NCC(=O)N | ||
| Standard InChIKey | MLSVJHOYXJGGTR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C46H64N14O12S2.C2H4O2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25;1-2(3)4/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52);1H3,(H,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Desmopressin(DDAVP) acetate is the synthetic analogue of the antidiuretic hormone arginine vasopressin.
IC50 Value:
Target: Vasopressin Receptor
The antidiuretic properties of desmopressin have led to its use in polyuric conditions including primary nocturnal enuresis, nocturia, and diabetes insipidus. Desmopressin works by limiting the amount of water that is eliminated in the urine. Desmopressin binds to V2 receptors in renal collecting ducts, increasing water reabsorption. It also stimulates release of von Willebrand factor from endothelial cells by acting on the V2 receptor. References: | |||||

Desmopressin Acetate Dilution Calculator

Desmopressin Acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8855 mL | 4.4276 mL | 8.8553 mL | 17.7106 mL | 22.1382 mL |
| 5 mM | 0.1771 mL | 0.8855 mL | 1.7711 mL | 3.5421 mL | 4.4276 mL |
| 10 mM | 0.0886 mL | 0.4428 mL | 0.8855 mL | 1.7711 mL | 2.2138 mL |
| 50 mM | 0.0177 mL | 0.0886 mL | 0.1771 mL | 0.3542 mL | 0.4428 mL |
| 100 mM | 0.0089 mL | 0.0443 mL | 0.0886 mL | 0.1771 mL | 0.2214 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Desmopressin acetate is the synthetic analogue of the antidiuretic hormone arginine vasopressin. The antidiuretic properties of desmopressin have led to its use in polyuric conditions including primary nocturnal enuresis, nocturia, and diabetes insipidus. Desmopressin works by limiting the amount of water that is eliminated in the urine. Desmopressin binds to V2 receptors in renal collecting ducts, increasing water reabsorption. It also stimulates release of von Willebrand factor from endothelial cells by acting on the V2 receptor.
- (-)-p-Bromotetramisole Oxalate
Catalog No.:BCC5449
CAS No.:62284-79-1
- Ponceau S Staining Solution
Catalog No.:BCC8032
CAS No.:6226-79-5
- JLK 6
Catalog No.:BCC2343
CAS No.:62252-26-0
- 1-Hydroxy-9-medroxycanthin-6-one
Catalog No.:BCN3103
CAS No.:622408-85-9
- Bifemelane hydrochloride
Catalog No.:BCC6786
CAS No.:62232-46-6
- 4-Hydroxy-2,6,6-trimethyl-1-cyclohexenecarboxylic acid
Catalog No.:BCN1394
CAS No.:62218-55-7
- 3-O-Methylducheside A
Catalog No.:BCN4153
CAS No.:62218-23-9
- alpha-Viniferin
Catalog No.:BCN4152
CAS No.:62218-13-7
- epsilon-Viniferin
Catalog No.:BCN4151
CAS No.:62218-08-0
- 1,2,3-Triacetyl-5-deoxy-D-ribose
Catalog No.:BCC8408
CAS No.:62211-93-2
- 12-O-Methylcarnosic acid
Catalog No.:BCN7655
CAS No.:62201-71-2
- Hordenine sulfate
Catalog No.:BCC8184
CAS No.:622-64-0
- 4-Hydroxybenzyl alcohol
Catalog No.:BCN4154
CAS No.:623-05-2
- H-Gly-OEt.HCl
Catalog No.:BCC2950
CAS No.:623-33-6
- H-Tyr(Me)-OH
Catalog No.:BCC3133
CAS No.:6230-11-1
- Rutaevin 7-acetate
Catalog No.:BCN7991
CAS No.:62306-81-4
- 5-Hydroxy-2-pyrrolidinone
Catalog No.:BCN4155
CAS No.:62312-55-4
- Ki20227
Catalog No.:BCC1678
CAS No.:623142-96-1
- Arjunglucoside I
Catalog No.:BCN8259
CAS No.:62319-70-4
- Lappaol A
Catalog No.:BCN8280
CAS No.:62333-08-8
- H-Glu(OtBu)-OMe.HCl
Catalog No.:BCC2935
CAS No.:6234-01-1
- Oxyntomodulin
Catalog No.:BCC5874
CAS No.:62340-29-8
- Isotschimgin
Catalog No.:BCN4156
CAS No.:62356-47-2
- Arjunglucoside II
Catalog No.:BCN6395
CAS No.:62369-72-6
Buccally Administered Intranasal Desmopressin Acetate for the Treatment of Neurogenic Diabetes Insipidus in Infancy.[Pubmed:27011115]
J Clin Endocrinol Metab. 2016 May;101(5):2084-8.
CONTEXT: The treatment of neurogenic diabetes insipidus (DI) in infancy is challenging and complicated by fluid overload and dehydration. Therapy with subcutaneous (SC), intranasal (IN), or oral tablet Desmopressin Acetate (1-desamino-8-D-arginine vasopressin [DDAVP]) remains difficult to titrate in infants. OBJECTIVE: Assess the efficacy and safety of buccally administered IN DDAVP for the management of infants with neurogenic DI. DESIGN, SETTING, PARTICIPANTS, AND INTERVENTION: Retrospective review of clinical and laboratory data of 15 infants (mean age, 4.5 mo) with neurogenic DI treated at a tertiary care center. Treatment was with diluted IN DDAVP formulation (10 mcg/mL) administered buccally via a tuberculin syringe to the buccal mucosa. RESULTS: After initial DDAVP titration of 2-3 days, IN DDAVP doses ranged from 1 to 5 mcg twice daily given buccally. Mean serum sodium concentration at DI diagnosis was 159 +/- 6.6 mmol/L (range, 151-178) and improved to 142 +/- 3.5 mmol/L (range, 137-147) with the buccally administered IN DDAVP. Normal sodium concentrations were established without major fluctuations. Serum sodium was then maintained in the outpatient setting at a mean of 145.7 +/- 4.8 mmol/L (mean duration of follow-up, 11 mo). CONCLUSIONS: Buccally administered IN formulation of DDAVP provides a practical and safe treatment alternative for neurogenic DI in infancy. Our approach avoided severe hypo- and hypernatremia during DDAVP titration and ongoing outpatient management of DI. The possibility for smaller dosage increments and ease of administration make IN DDAVP administered buccally preferable over other DDAVP treatment options in infants.
[Effects of desmopressin acetate and pituitrin on proliferation, contraction, and secretion of hepatic stellate cells].[Pubmed:27788702]
Zhonghua Gan Zang Bing Za Zhi. 2016 Aug 20;24(8):569-574.
Objective: To investigate the effects of Desmopressin Acetate and pituitrin on the proliferation, contraction, and secretion of hepatic stellate cells (HSCs). Methods: The human HSC cell line LX-2 was selected as the research model. And three groups were designed: blank control group, Desmopressin Acetate group (three subgroups: 1x10(-10)mol/L, 1x10(-9)mol/L, and 1x10(-8)mol/L Desmopressin Acetate), and pituitrin group (three subgroups: 0.1 U/L, 1.0 U/L, and 10.0 U/L pituitrin). Water-soluble tetrazolium salt (WST)-1 assay was used to evaluate cell proliferation; collagen gel contraction assay was used to assess cell contraction; enzyme-linked immunosorbent assay (ELISA) was used to identify cell secretion. The data was subjected to one-way analysis of variance. Results: (1) The results of WST-1 assay showed that the values of A450in three Desmopressin Acetate subgroups (1x10(-10)mol/L, 1x10(-9)mol/L, and 1x10(-8)mol/L) were 0.459+/-0.017, 0.467+/-0.024, and 0.436+/-0.015, respectively. And the values of A450 in three pituitrin subgroups (0.1 U/L, 1.0 U/L, and 10.0 U/L) were 0.495+/-0.011, 0.507+/-0.015, and 0.501+/-0.009, respectively. Compared with the control group, the Desmopressin Acetate at high concentration significantly inhibited the cell proliferation (P< 0.05), but the pituitrin at three different concentrations significantly promoted the cell proliferation (P< 0.05). (2) The collagen gel area ratios in three Desmopressin Acetate subgroups (1x10(-10)mol/L, 1x10(-9)mol/L, and 1x10(-8)mol/L) were 77.07+/-4.42, 75.85+/-3.70, and 72.74+/-3.92, respectively. And the collagen gel area ratios in three pituitrin subgroups (0.1 U/L, 1.0 U/L, and 10.0 U/L) were 57.83+/-3.96, 50.28+/-6.69, and 43.56+/-7.68, respectively. Compared with the control group, the pituitrin at three different concentrations significantly reduced the collagen gel area (P< 0.01). (3) The matrix metalloproteinase(MMP)-2 concentrations in three Desmopressin Acetate subgroups (1x10(-10)mol/L, 1x10(-9)mol/L, and 1x10(-8)mol/L) were 13.321+/-0.098, 12.230+/-0.153, and 12.061+/-0.126, respectively. And the MMP-2 concentrations in three pituitrin subgroups (0.1 U/L, 1.0 U/L, and 10.0 U/L) were 12.899+/-0.150, 13.662+/-0.152, and 13.698+/-0.119, respectively. Compared with the control group, the Desmopressin Acetate at low concentration significantly increased the secretion of MMP-2 (P< 0.01); the Desmopressin Acetate at high concentration significantly decreased the MMP-2 concentration (P< 0.05); the pituitrin at three different concentrations significantly increased the MMP-2 concentration (P< 0.01). The transforming growth factor-beta 1 (TGF-beta1) concentrations in three Desmopressin Acetate subgroups (1x10(-10)mol/L, 1x10(-9)mol/L, and 1x10(-8)mol/L) were 5.233+/-0.102, 17.749+/-0.188, and 36.060+/-0.227, respectively. And the TGF-beta1 concentrations in three pituitrin subgroups (0.1 U/L, 1.0 U/L, and 10.0 U/L) were 15.615+/-0.099, 38.460+/-0.209, and 49.053+/-0.115, respectively. Compared with the control group, Desmopressin Acetate and pituitrin significantly promoted the secretion of TGF-beta1 in a concentration-dependent manner (P< 0.01) and pituitrin had a stronger effect than Desmopressin Acetate (P< 0.01). Desmopressin Acetate and pituitrin had no effect on the secretion of the collagenase type I and III (P> 0.05). Conclusion: Desmopressin Acetate and pituitrin can induce the changes in the function and morphology of HSCs and may increase vascular resistance in the hepatic sinus. However, Desmopressin Acetate has less influence on HSCs than pituitrin.
Utilization and Effectiveness of Desmopressin Acetate After Cardiac Surgery Supplemented With Point-of-Care Hemostatic Testing: A Propensity-Score-Matched Analysis.[Pubmed:28169116]
J Cardiothorac Vasc Anesth. 2017 Jun;31(3):883-895.
OBJECTIVES: To explore the utilization pattern and hemostatic effectiveness of Desmopressin Acetate (DDAVP) supplemented with point-of-care (POC) hemostatic testing in contemporary cardiac surgery. DESIGN: Retrospective, observational study. SETTING: Single quaternary care university hospital. PARTICIPANTS: The study comprised 2,468 consecutive patients undergoing cardiac surgery-1,237 before and 1,231 after the introduction of POC testing. INTERVENTIONS: The incidence of DDAVP administration during the year before (2012) and after (2013) the initiation of POC-based viscoelastic (ROTEM; Tem International GmBH, Munich, Germany) and platelet function (Plateletworks; Helena Laboratories, Beaumont, TX) testing was determined. Propensity-score matching was used to examine the association between DDAVP administration and major bleeding during each time period. MEASUREMENTS AND MAIN RESULTS: DDAVP was administered more than twice as often after POC implementation (41% v 20%, p<0.001). Major bleeding was defined based on the universal definition of perioperative bleeding in adult cardiac surgery. Propensity matching identified 224 well-balanced pairs of DDAVP recipients and control patients before and 298 such pairs after the implementation of POC testing. After adjusting for matched data, DDAVP administration was associated with 1.70 (95% confidence interval 1.25-2.32, p<0.001) and 1.51 (95% confidence interval 1.15-1.98, p = 0.003) increases in the odds of major bleeding before and after the initiation of POC testing, respectively. CONCLUSIONS: Clinicians should be cognizant of the potential for increased use of DDAVP despite limited evidence of benefit in contemporary cardiac anesthesia practice supplemented with POC-based hemostatic testing.
Influence of different stabilizers on the encapsulation of desmopressin acetate into PLGA nanoparticles.[Pubmed:28011093]
Eur J Pharm Biopharm. 2017 Sep;118:48-55.
To address targeting and bioavailability issues of peptidic drugs like desmopressin, the encapsulation into nanoparticles (NP) has become standard in pharmaceutics. This study investigated the encapsulation of desmopressin into PLGA NP by the use of pharmaceutically common stabilizers as a precursor to future, optional targeting and bioavailability experiments. Polymer dry weights were measured by freeze drying and thermo gravimetric analysis (TGA). Particle sizes (ranging between 105 and 130nm, PDI<0.1) and zeta potentials (-35 to -45mV) were analyzed with Dynamic Light Scattering (DLS) and Laser-Doppler-Anemometry (LDA) respectively. Highest loading efficiencies, quantified by RP-HPLC, were achieved with Pluronic F-68 as stabilizer of the inner aqueous phase (1.16+/-0.07mug desmopressin/mg PLGA) and were significantly higher than coating approaches and approaches without stabilizer (0.74+/-0.01mug/mg). Optimized nanoformulations are thus in competition with the concentration of commercial non-nanoparticulate desmopressin products. Stability of desmopressin after the process was evaluated by HPLC peak purity analysis (diode array detector) and by mass spectrometry. Desmopressin was shown to remain intact during the whole process; however, despite these very good results the encapsulation efficiency turned out to be a bottle neck and makes the system a challenge for potential applications.


