H-Tyr(Me)-OHCAS# 6230-11-1 |
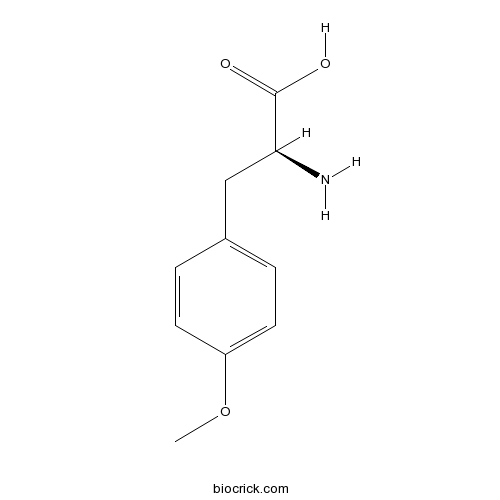
- H-D-Phe(4-OMe)-OH
Catalog No.:BCC2633
CAS No.:39878-65-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6230-11-1 | SDF | Download SDF |
| PubChem ID | 2723935 | Appearance | Powder |
| Formula | C10H13NO3 | M.Wt | 195.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-amino-3-(4-methoxyphenyl)propanoic acid | ||
| SMILES | COC1=CC=C(C=C1)CC(C(=O)O)N | ||
| Standard InChIKey | GEYBMYRBIABFTA-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C10H13NO3/c1-14-8-4-2-7(3-5-8)6-9(11)10(12)13/h2-5,9H,6,11H2,1H3,(H,12,13)/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

H-Tyr(Me)-OH Dilution Calculator

H-Tyr(Me)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.123 mL | 25.6148 mL | 51.2295 mL | 102.459 mL | 128.0738 mL |
| 5 mM | 1.0246 mL | 5.123 mL | 10.2459 mL | 20.4918 mL | 25.6148 mL |
| 10 mM | 0.5123 mL | 2.5615 mL | 5.123 mL | 10.2459 mL | 12.8074 mL |
| 50 mM | 0.1025 mL | 0.5123 mL | 1.0246 mL | 2.0492 mL | 2.5615 mL |
| 100 mM | 0.0512 mL | 0.2561 mL | 0.5123 mL | 1.0246 mL | 1.2807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Tyr(Me)-OH
- H-Gly-OEt.HCl
Catalog No.:BCC2950
CAS No.:623-33-6
- 4-Hydroxybenzyl alcohol
Catalog No.:BCN4154
CAS No.:623-05-2
- Desmopressin Acetate
Catalog No.:BCC1526
CAS No.:62288-83-9
- (-)-p-Bromotetramisole Oxalate
Catalog No.:BCC5449
CAS No.:62284-79-1
- Ponceau S Staining Solution
Catalog No.:BCC8032
CAS No.:6226-79-5
- JLK 6
Catalog No.:BCC2343
CAS No.:62252-26-0
- 1-Hydroxy-9-medroxycanthin-6-one
Catalog No.:BCN3103
CAS No.:622408-85-9
- Bifemelane hydrochloride
Catalog No.:BCC6786
CAS No.:62232-46-6
- 4-Hydroxy-2,6,6-trimethyl-1-cyclohexenecarboxylic acid
Catalog No.:BCN1394
CAS No.:62218-55-7
- 3-O-Methylducheside A
Catalog No.:BCN4153
CAS No.:62218-23-9
- alpha-Viniferin
Catalog No.:BCN4152
CAS No.:62218-13-7
- epsilon-Viniferin
Catalog No.:BCN4151
CAS No.:62218-08-0
- Rutaevin 7-acetate
Catalog No.:BCN7991
CAS No.:62306-81-4
- 5-Hydroxy-2-pyrrolidinone
Catalog No.:BCN4155
CAS No.:62312-55-4
- Ki20227
Catalog No.:BCC1678
CAS No.:623142-96-1
- Arjunglucoside I
Catalog No.:BCN8259
CAS No.:62319-70-4
- Lappaol A
Catalog No.:BCN8280
CAS No.:62333-08-8
- H-Glu(OtBu)-OMe.HCl
Catalog No.:BCC2935
CAS No.:6234-01-1
- Oxyntomodulin
Catalog No.:BCC5874
CAS No.:62340-29-8
- Isotschimgin
Catalog No.:BCN4156
CAS No.:62356-47-2
- Arjunglucoside II
Catalog No.:BCN6395
CAS No.:62369-72-6
- Kadsuric acid
Catalog No.:BCN4157
CAS No.:62393-88-8
- Alboctalol
Catalog No.:BCN4158
CAS No.:62394-00-7
- Cinnzeylanol
Catalog No.:BCN4159
CAS No.:62394-04-1
Role for engagement of beta-arrestin2 by the transactivated EGFR in agonist-specific regulation of delta receptor activation of ERK1/2.[Pubmed:26211551]
Br J Pharmacol. 2015 Oct;172(20):4847-63.
BACKGROUND AND PURPOSE: beta-Arrestins function as signal transducers linking GPCRs to ERK1/2 signalling either by scaffolding members of ERK1/2s cascades or by transactivating receptor tyrosine kinases through Src-mediated release of transactivating factor. Recruitment of beta-arrestins to the activated GPCRs is required for ERK1/2 activation. Our previous studies showed that delta receptors activate ERK1/2 through a beta-arrestin-dependent mechanism without inducing beta-arrestin binding to the delta receptors. However, the precise mechanisms involved remain to be established. EXPERIMENTAL APPROACH: ERK1/2 activation by delta receptor ligands was assessed using HEK293 cells in vitro and male Sprague Dawley rats in vivo. Immunoprecipitation, immunoblotting, siRNA transfection, intracerebroventricular injection and immunohistochemistry were used to elucidate the underlying mechanism. KEY RESULTS: We identified a new signalling pathway in which recruitment of beta-arrestin2 to the EGFR rather than delta receptor was required for its role in delta receptor-mediated ERK1/2 activation in response to H-Tyr-Tic-Phe-Phe-OH (TIPP) or morphine stimulation. Stimulation of the delta receptor with ligands leads to the phosphorylation of PKCdelta, which acts upstream of EGFR transactivation and is needed for the release of the EGFR-activating factor, whereas beta-arrestin2 was found to act downstream of the EGFR transactivation. Moreover, we demonstrated that coupling of the PKCdelta/EGFR/beta-arrestin2 transactivation pathway to delta receptor-mediated ERK1/2 activation was ligand-specific and the Ser(363) of delta receptors was crucial for ligand-specific implementation of this ERK1/2 activation pathway. CONCLUSIONS AND IMPLICATIONS: The delta receptor-mediated activation of ERK1/2 is via ligand-specific transactivation of EGFR. This study adds new insights into the mechanism by which delta receptors activate ERK1/2.
Effects of Tyroserleutide on phosphatidylinositol 3'-kinase/AKT pathway in human hepatocellular carcinoma cell.[Pubmed:24147456]
J Drug Target. 2014 Feb;22(2):146-55.
Tyroserleutide (YSL) is an active, low-molecular-weight polypeptide with in vitro and in vivo anticancer effects on human hepatocellular carcinoma BEL-7402 cells. In this study, we studied the effects of YSL on PI3K/AKT in the BEL-7402 cells to explore its anti-tumor mechanism. Results showed that YSL could up-regulate the mRNA and protein expression of tumor suppressor PTEN and increase their activities, meanwhile inhibited the mRNA and protein expression of oncogene AKT and decreased the kinase activities of AKT and PDK1. The resuming balance effect of YSL between PTEN and AKT could prevent the transmission of tumor cell proliferation signals in the PI3K/AKT pathway. Inhibition of AKT would change the status of downstream effectors in the PI3K/AKT pathway: (1) inhibition of AKT up-regulated expression of cell cycle regulatory factors of downstream - P21 and P27 which repressed cell cycle and inhibited proliferation of tumor cells. (2) Inhibition of AKT decreased the phosphorylation level of MDM2, and then increased the protein level of P53 which would accelerate death proceeding of tumor cells. (3) Inactivation of AKT removed its inhibition effect on phosphorylation of Bad, which might decrease protein level of apoptosis inhibitor Bcl-2 and Bcl-XL, damaging mitochondria of tumor cells and inducing apoptosis.
Two delta opioid receptor subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor.[Pubmed:28645621]
Neuropharmacology. 2017 Sep 1;123:420-432.
The mu and delta opioid receptors (MOR and DOR) are highly homologous members of the opioid family of GPCRs. There is evidence that MOR and DOR interact, however the extent to which these interactions occur in vivo and affect synaptic function is unknown. There are two stable DOR subtypes: DPDPE sensitive (DOR1) and deltorphin II sensitive (DOR2); both agonists are blocked by DOR selective antagonists. Robust motivational effects are produced by local actions of both MOR and DOR ligands in the ventral tegmental area (VTA). Here we demonstrate that a majority of both dopaminergic and non-dopaminergic VTA neurons express combinations of functional DOR1, DOR2, and/or MOR, and that within a single VTA neuron, DOR1, DOR2, and MOR agonists can differentially couple to downstream signaling pathways. As reported for the MOR agonist DAMGO, DPDPE and deltorphin II produced either a predominant K(+) dependent hyperpolarization or a Cav2.1 mediated depolarization in different neurons. In some neurons DPDPE and deltorphin II produced opposite responses. Excitation, inhibition, or no effect by DAMGO did not predict the response to DPDPE or deltorphin II, arguing against a MOR-DOR interaction generating DOR subtypes. However, in a subset of VTA neurons the DOR antagonist TIPP-Psi augmented DAMGO responses; we also observed DPDPE or deltorphin II responses augmented by the MOR selective antagonist CTAP. These findings directly support the existence of two independent, stable forms of the DOR, and show that MOR and DOR can interact in some neurons to alter downstream signaling.
N-terminal guanidinylation of TIPP (Tyr-Tic-Phe-Phe) peptides results in major changes of the opioid activity profile.[Pubmed:23932788]
Bioorg Med Chem Lett. 2013 Sep 15;23(18):5082-5.
Derivatives of peptides of the TIPP (Tyr-Tic-Phe-Phe; Tic=1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) family containing a guanidino (Guan) function in place of the N-terminal amino group were synthesized in an effort to improve their blood-brain barrier permeability. Unexpectedly, N-terminal amidination significantly altered the in vitro opioid activity profiles. Guan-analogues of TIPP-related delta opioid antagonists showed delta partial agonist or mixed delta partial agonist/mu partial agonist activity. Guanidinylation of the mixed mu agonist/delta antagonists H-Dmt-Tic-Phe-Phe-NH2 (DIPP-NH2) and H-Dmt-TicPsi[CH2NH]Phe-Phe-NH2 (DIPP-NH2[Psi]) converted them to mixed mu agonist/delta agonists. A docking study revealed distinct positioning of DIPP-NH2 and Guan-DIPP-NH2 in the delta receptor binding site. Lys(3)-analogues of DIPP-NH2 and DIPP-NH2[Psi] (guanidinylated or non-guanidinylated) turned out to be mixed mu/kappa agonists with delta antagonist-, delta partial agonist- or delta full agonist activity. Compounds with some of the observed mixed opioid activity profiles have therapeutic potential as analgesics with reduced side effects or for treatment of cocaine addiction.
A G protein-coupled receptor (GPCR) in red: live cell imaging of the kappa opioid receptor-tdTomato fusion protein (KOPR-tdT) in neuronal cells.[Pubmed:23856011]
J Pharmacol Toxicol Methods. 2013 Nov-Dec;68(3):340-5.
INTRODUCTION: In contrast to green fluorescent protein and variants (GFPs), red fluorescent proteins (RFPs) have rarely been employed for the generation of GPCR fusion proteins, likely because of formation of aggregates and cell toxicity of some RFPs. Among all the RFPs, tdTomato (tdT), one of the non-aggregating RFP, has the highest brightness score (about 3 times that of eGFP) and unsurpassed photostability. METHODS: We fused tdT to the KOPR C-terminus. The KOPR-tdT cDNA construct was transfected into a Neuro2A mouse neuroblastoma cell line (Neuro2A cells) and rat cortical primary neurons for characterization of pharmacological properties and imaging studies on KOPR trafficking. RESULTS: KOPR-tdT retained KOPR properties (cell surface expression, ligand binding, agonist-induced signaling and internalization) when expressed in Neuro2A cells and rat primary cortical neurons. Live cell imaging of KOPR-tdT enables visualization of the time course of agonist-induced internalization of KOPR in real time for 60 min, without photobleaching and apparent cell toxicity. U50,488H-induced KOPR internalization occurred as early as 4min and plateaued at about 30 min. A unique pattern of internalized KOPR in processes of primary neurons was induced by U50,488H. DISCUSSION: tdT is an alternative to, or even a better tool than, GFPs for fusion to GPCR for trafficking studies, because tdT has higher brightness and thus better resolution and less photobleaching problems due to the reduced laser power used. It also has advantages associated with its longer-wavelength emission including spectral separation from autofluorescence and GFPs, reduced cell toxicity that the laser may impose, and greater tissue penetration. These advantages of tdT over GPFs may be critical for live cell imaging studies of GPCRs in vitro and for studying GPCRs in vivo because of their low abundance.


