Quercetin-3-O-sophorosideCAS# 18609-17-1 |
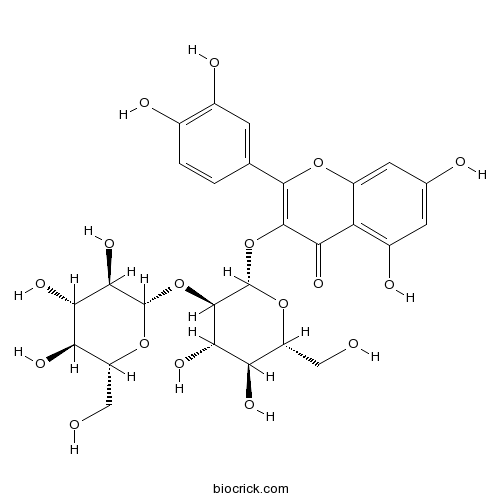
- Quercetin 3-glucosyl-(1->2)-galactoside
Catalog No.:BCX0122
CAS No.:95043-15-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18609-17-1 | SDF | Download SDF |
| PubChem ID | 5282166 | Appearance | Yellowish powder |
| Formula | C27H30O17 | M.Wt | 626.51 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Baimaside; 3,3',4',5,7-Pentahydroxyflavone 3-sophoroside | ||
| Solubility | Soluble in methanol; slightly soluble in water | ||
| Chemical Name | 3-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | ||
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)OC4C(C(C(C(O4)CO)O)O)OC5C(C(C(C(O5)CO)O)O)O)O)O | ||
| Standard InChIKey | RDUAJIJVNHKTQC-UJECXLDQSA-N | ||
| Standard InChI | InChI=1S/C27H30O17/c28-6-14-17(34)20(37)22(39)26(41-14)44-25-21(38)18(35)15(7-29)42-27(25)43-24-19(36)16-12(33)4-9(30)5-13(16)40-23(24)8-1-2-10(31)11(32)3-8/h1-5,14-15,17-18,20-22,25-35,37-39H,6-7H2/t14-,15-,17-,18-,20+,21+,22-,25-,26+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Quercetin-3-O-sophoroside is the natural ligand of Bet v 1. |
| Structure Identification | J Pharm Biomed Anal. 2015 Mar 25;107:273-9.Application of mixed cloud point extraction for the analysis of six flavonoids in Apocynum venetum leaf samples by high performance liquid chromatography.[Pubmed: 25625477]
|

Quercetin-3-O-sophoroside Dilution Calculator

Quercetin-3-O-sophoroside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5961 mL | 7.9807 mL | 15.9614 mL | 31.9229 mL | 39.9036 mL |
| 5 mM | 0.3192 mL | 1.5961 mL | 3.1923 mL | 6.3846 mL | 7.9807 mL |
| 10 mM | 0.1596 mL | 0.7981 mL | 1.5961 mL | 3.1923 mL | 3.9904 mL |
| 50 mM | 0.0319 mL | 0.1596 mL | 0.3192 mL | 0.6385 mL | 0.7981 mL |
| 100 mM | 0.016 mL | 0.0798 mL | 0.1596 mL | 0.3192 mL | 0.399 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Deacetylnimbin
Catalog No.:BCN4684
CAS No.:18609-16-0
- Kakuol
Catalog No.:BCN6455
CAS No.:18607-90-4
- RS 79948 hydrochloride
Catalog No.:BCC6876
CAS No.:186002-54-0
- AMD 3465
Catalog No.:BCC5181
CAS No.:185991-24-6
- AMD 3465 hexahydrobromide
Catalog No.:BCC5182
CAS No.:185991-07-5
- H-Ile-OMe.HCl
Catalog No.:BCC2964
CAS No.:18598-74-8
- H-Cys-OMe.HCl
Catalog No.:BCC2905
CAS No.:18598-63-5
- Echinulin
Catalog No.:BCN1157
CAS No.:1859-87-6
- Pterodontic acid
Catalog No.:BCN1156
CAS No.:185845-89-0
- 2,3-Dihydroxypterodontic acid
Catalog No.:BCN1155
CAS No.:185821-32-3
- Viroallosecurinine
Catalog No.:BCN6743
CAS No.:1857-30-3
- BMS 961
Catalog No.:BCC7680
CAS No.:185629-22-5
- 11alpha,12alpha-Epoxy-3beta,23-dihydroxy-30-norolean-20(29)-en-28,13beta-olide
Catalog No.:BCN1515
CAS No.:186140-36-3
- (-)-Anonaine
Catalog No.:BCN8235
CAS No.:1862-41-5
- H-Dapa-OH.HBr
Catalog No.:BCC2668
CAS No.:18635-45-5
- enantio-7(11)-Eudesmen-4-ol
Catalog No.:BCN1158
CAS No.:186374-63-0
- CP-91149
Catalog No.:BCC3757
CAS No.:186392-40-5
- CP 316819
Catalog No.:BCC6039
CAS No.:186392-43-8
- Psoralidin
Catalog No.:BCN5414
CAS No.:18642-23-4
- Actein
Catalog No.:BCN1159
CAS No.:18642-44-9
- Alisol B
Catalog No.:BCN3364
CAS No.:18649-93-9
- Zibotentan (ZD4054)
Catalog No.:BCC2524
CAS No.:186497-07-4
- LY 344864
Catalog No.:BCC1716
CAS No.:186544-26-3
- 1,2-Bis(3-indenyl)ethane
Catalog No.:BCC8413
CAS No.:18657-57-3
New flavonol glycosides from the leaves of Triantha japonica and Tofieldia nuda.[Pubmed:24273859]
Nat Prod Commun. 2013 Sep;8(9):1251-4.
Two new flavonol glycosides were isolated from the leaves of Triantha japonica, together with eight known flavonols, kaempferol 3-O-sophoroside, kaempferol 3-O-sambubioside, kaempferol 3-O-glucosyl-(1 --> 2)-[glucosyl-(1 --> 6)-glucoside], quercetin 3-O-sophoroside, quercetin 3-O-sambubioside, isorhamnetin 3-O-glucoside, isorhamnetin 3-O-sophoroside and isorhamnetin 3-O-sambubioside. The new compounds were identified as kaempferol 3-O-beta-xylopyranosyl-(1 --> 2)-[beta-glucopyranosyl-(1 --> 6)-beta-glucopyranoside] (1) and isorhamnetin 3-O-beta-xylopyranosyl-(1 --> 2)-[beta-glucopyranosyl-(1 --> 6)-beta-glucopyranoside] (3) by UV, LC-MS, acid hydrolysis, and 1H and 13C NMR spectroscopy. Another two new flavonol glycosides were isolated from theleaves of Tofieldia nuda, and identified as kaempferol 3-O-beta-glucopyranosyl-(1 --> 2)-[beta-glucopyranosyl-(1 --> 6)-beta-galactopyranoside] (4) and quercetin 3-O-beta-glucopyranosyl-(1 --> 2)-[beta-glucopyranosyl-(1 --> 6)-beta-galactopyranoside] (5). Though the genera Triantha and Tofieldia were treated as Tofieldia sensu lato, they were recently divided into two genera. It was shown by this survey that their flavonoid composition were also different to each other.
Application of mixed cloud point extraction for the analysis of six flavonoids in Apocynum venetum leaf samples by high performance liquid chromatography.[Pubmed:25625477]
J Pharm Biomed Anal. 2015 Mar 25;107:273-9.
A simple, inexpensive and efficient method based on the mixed cloud point extraction (MCPE) combined with high performance liquid chromatography was developed for the simultaneous separation and determination of six flavonoids (rutin, hyperoside, Quercetin-3-O-sophoroside, isoquercitrin, astragalin and quercetin) in Apocynum venetum leaf samples. The non-ionic surfactant Genapol X-080 and cetyl-trimethyl ammonium bromide (CTAB) was chosen as the mixed extracting solvent. Parameters that affect the MCPE processes, such as the content of Genapol X-080 and CTAB, pH, salt content, extraction temperature and time were investigated and optimized. Under the optimized conditions, the calibration curve for six flavonoids were all linear with the correlation coefficients greater than 0.9994. The intra-day and inter-day precision (RSD) were below 8.1% and the limits of detection (LOD) for the six flavonoids were 1.2-5.0 ng mL(-1) (S/N=3). The proposed method was successfully used to separate and determine the six flavonoids in A. venetum leaf samples.


