Apoptosis Activator 2Indoledione caspase activator, cell-permeable CAS# 79183-19-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79183-19-0 | SDF | Download SDF |
| PubChem ID | 1901244 | Appearance | Powder |
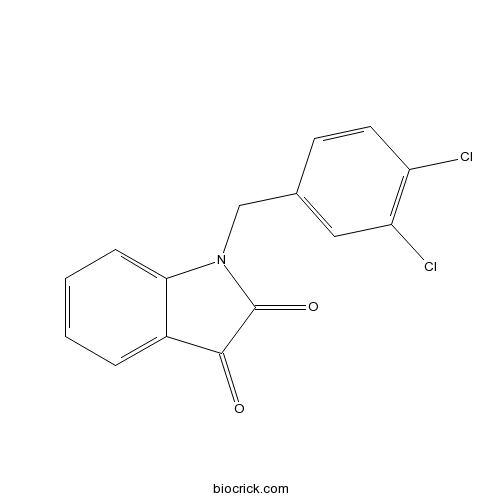
| Formula | C15H9Cl2NO2 | M.Wt | 306.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 1-(3,4-Dichlorobenzyl)-1H-indole-2,3-dione | ||
| Solubility | Soluble to 5 mM in ethanol and to 100 mM in DMSO | ||
| Chemical Name | 1-[(3,4-dichlorophenyl)methyl]indole-2,3-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C(=O)N2CC3=CC(=C(C=C3)Cl)Cl | ||
| Standard InChIKey | KGRJPLRFGLMQMV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H9Cl2NO2/c16-11-6-5-9(7-12(11)17)8-18-13-4-2-1-3-10(13)14(19)15(18)20/h1-7H,8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Apoptosis activator; promotes the cytochrome c-dependent oligomerization of Apaf-1 into the mature apoptosome. Increases procaspase-9 processing and subsequent caspase-3 activation. Induces apoptosis in tumor cells (IC50 = 4 - 9 μM for leukemia cells) with weak or no effect on normal cell lines or those defective/deficient in Apaf-1, caspase-9 or caspase-3 activity (IC50 > 40 μM). |

Apoptosis Activator 2 Dilution Calculator

Apoptosis Activator 2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2669 mL | 16.3345 mL | 32.6691 mL | 65.3381 mL | 81.6727 mL |
| 5 mM | 0.6534 mL | 3.2669 mL | 6.5338 mL | 13.0676 mL | 16.3345 mL |
| 10 mM | 0.3267 mL | 1.6335 mL | 3.2669 mL | 6.5338 mL | 8.1673 mL |
| 50 mM | 0.0653 mL | 0.3267 mL | 0.6534 mL | 1.3068 mL | 1.6335 mL |
| 100 mM | 0.0327 mL | 0.1633 mL | 0.3267 mL | 0.6534 mL | 0.8167 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Apoptosis Activator 2 is a small molecule apoptosis activator with IC50 value of about 4μM [1].
Apoptosis Activator 2 is a cell permeable compound that promotes apoptosis by activating caspases in a cytochrome c- and Apaf-1-dependent manner. 20μM Apoptosis Activator 2 increases the fraction of Apaf-1 in the apoptosome when there is 0.15μM cytochrome c. It is found to induce the oligomerization of Apaf-1. Through the induction of apoptosis, Apoptosis Activator 2 notably induces caspase-3 activation, PARP cleavage and DNA fragmentation. Moreover, this induction of apoptosis only occurs in tumor cell lines (such as breast, lung, colon, and epidermal cancer cell lines). Normal cell lines are resistant to Apoptosis Activator 2 [1, 2].
References:
[1] Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7533-8.
[2] Nguyen TV, Jayaraman A, Quaglino A, Pike CJ. Androgens selectively protect against apoptosis in hippocampal neurones. J Neuroendocrinol. 2010 Sep;22(9):1013-22.
- Diammonium glycyrrhizinate
Catalog No.:BCN7145
CAS No.:79165-06-3
- A-7 hydrochloride
Catalog No.:BCC6625
CAS No.:79127-24-5
- Hannokinol
Catalog No.:BCN6514
CAS No.:79120-40-4
- Lariciresinol acetate
Catalog No.:BCN4577
CAS No.:79114-77-5
- Polygalasaponin XXXI
Catalog No.:BCN2857
CAS No.:79103-90-5
- (-)-Corlumine
Catalog No.:BCN6632
CAS No.:79082-64-7
- Boc-Serinol(Bzl)
Catalog No.:BCC2706
CAS No.:79069-15-1
- Boc-Valinol
Catalog No.:BCC2695
CAS No.:79069-14-0
- Boc-Alaninol
Catalog No.:BCC2730
CAS No.:79069-13-9
- D-AP5
Catalog No.:BCC6553
CAS No.:79055-68-8
- L-AP5
Catalog No.:BCC6554
CAS No.:79055-67-7
- β-Estradiol - d3
Catalog No.:BCC5365
CAS No.:79037-37-9
- 7-Hydroxyaristolochic acid A
Catalog No.:BCN2659
CAS No.:79185-75-4
- Cyclosporine
Catalog No.:BCC2559
CAS No.:79217-60-0
- SB-334867 free base
Catalog No.:BCC5200
CAS No.:792173-99-0
- SA 47
Catalog No.:BCC6289
CAS No.:792236-07-8
- Deoxyandrographolide
Catalog No.:BCN2660
CAS No.:79233-15-1
- Azelastine HCl
Catalog No.:BCC4537
CAS No.:79307-93-0
- Streptenol E
Catalog No.:BCC8457
CAS No.:
- H-D-Glu-OBzl
Catalog No.:BCC2937
CAS No.:79338-14-0
- Cefixime
Catalog No.:BCC8907
CAS No.:79350-37-1
- AAL Toxin TA2
Catalog No.:BCN1738
CAS No.:79367-51-4
- AAL Toxin TA1
Catalog No.:BCN1733
CAS No.:79367-52-5
- BIBU 1361 dihydrochloride
Catalog No.:BCC7356
CAS No.:793726-84-8
The sGC activator inhibits the proliferation and migration, promotes the apoptosis of human pulmonary arterial smooth muscle cells via the up regulation of plasminogen activator inhibitor-2.[Pubmed:25704756]
Exp Cell Res. 2015 Mar 15;332(2):278-87.
BACKGROUND: Different types of pulmonary hypertension (PH) share the same process of pulmonary vascular remodeling, the molecular mechanism of which is not entirely clarified by far. The abnormal biological behaviors of pulmonary arterial smooth muscle cells (PASMCs) play an important role in this process. OBJECTIVES: We investigated the regulation of plasminogen activator inhibitor-2 (PAI-2) by the sGC activator, and explored the effect of PAI-2 on PASMCs proliferation, apoptosis and migration. METHODS: After the transfection with PAI-2 overexpression vector and specific siRNAs or treatment with BAY 41-2272 (an activator of sGC), the mRNA and protein levels of PAI-2 in cultured human PASMCs were detected, and the proliferation, apoptosis and migration of PASMCs were investigated. RESULTS: BAY 41-2272 up regulated the endogenous PAI-2 in PASMCs, on the mRNA and protein level. In PAI-2 overexpression group, the proliferation and migration of PASMCs were inhibited significantly, and the apoptosis of PASMCs was increased. In contrast, PAI-2 knockdown with siRNA increased PASMCs proliferation and migration, inhibited the apoptosis. CONCLUSIONS: PAI-2 overexpression inhibits the proliferation and migration and promotes the apoptosis of human PASMCs. Therefore, sGC activator might alleviate or reverse vascular remodeling in PH through the up-regulation of PAI-2.
Recombinant Human Erythropoietin Protects Myocardial Cells from Apoptosis via the Janus-Activated Kinase 2/Signal Transducer and Activator of Transcription 5 Pathway in Rats with Epilepsy.[Pubmed:26649078]
Curr Ther Res Clin Exp. 2015 Aug 4;77:90-8.
OBJECTIVE: To investigate the potential mechanisms underlying the protective effects of recombinant human erythropoietin (rhEPO) and carbamylated EPO (CEPO) against myocardial cell apoptosis in epilepsy. METHODS: Rats were given an intra-amygdala injection of kainic acid to induce epilepsy. Groups of rats were treated with rhEPO or CEPO before induction of epilepsy, whereas additional rats were given a caudal vein injection of AG490, a selective inhibitor of Janus kinase 2 (JAK2). At different time points after seizure onset, electroencephalogram changes were recorded, and myocardium samples were taken for the detection of myocardial cell apoptosis and expression of JAK2, signal transducer and activator of transcription 5 (STAT5), caspase-3, and bcl-xl mRNAs and proteins. RESULTS: Induction of epilepsy significantly enhanced myocardial cell apoptosis and upregulated the expression of caspase-3 and bcl-xl proteins and JAK2 and STAT5a at both the mRNA and protein levels. Pretreatment with either rhEPO or CEPO reduced the number of apoptotic cells, upregulated bcl-xl expression, and downregulated caspase-3 expression in the myocardium of epileptic rats. Both myocardial JAK2 and STAT5a mRNAs, as well as phosphorylated species of JAK2 and STAT5a, were upregulated in epileptic rats in response to rhEPO-but not to CEPO-pretreatment. AG490 treatment increased apoptosis, upregulated caspase-3 protein expression, and downregulated bcl-xl protein expression in the myocardium of epileptic rats. CONCLUSIONS: These results indicate that myocardial cell apoptosis may contribute to myocardial injury in epilepsy. EPO protects myocardial cells from apoptosis via the JAK2/STAT5 pathway in rats with experimental epilepsy, whereas CEPO exerts antiapoptotic activity perhaps via a pathway independent of JAK2/STAT5 signaling.
Apigenin Attenuates Atherogenesis through Inducing Macrophage Apoptosis via Inhibition of AKT Ser473 Phosphorylation and Downregulation of Plasminogen Activator Inhibitor-2.[Pubmed:25960827]
Oxid Med Cell Longev. 2015;2015:379538.
Macrophage survival is believed to be a contributing factor in the development of early atherosclerotic lesions. Dysregulated apoptosis of macrophages is involved in the inflammatory process of atherogenesis. Apigenin is a flavonoid that possesses various clinically relevant properties such as anti-inflammatory, antiplatelet, and antitumor activities. Here we showed that apigenin attenuated atherogenesis in apoE (-/-) mice in an in vivo test. In vitro experiments suggested that apigenin induced apoptosis of oxidized low density lipoprotein- (OxLDL-) loaded murine peritoneal macrophages (MPMs). Proteomic analysis showed that apigenin reduced the expression of plasminogen activator inhibitor 2 (PAI-2). PAI-2 has antiapoptotic effects in OxLDL-loaded MPMs. Enhancing PAI-2 expression significantly reduced the proapoptosis effects of apigenin. Molecular docking assay with AutoDock software predicted that residue Ser473 of Akt1 is a potential binding site for apigenin. Lentiviral-mediated overexpression of Akt1 wild type weakened the proapoptosis effect of apigenin in OxLDL-loaded MPMs. Collectively, apigenin executes its anti-atherogenic effects through inducing OxLDL-loaded MPMs apoptosis. The proapoptotic effects of apigenin were at least partly attributed to downregulation of PAI-2 through suppressing phosphorylation of AKT at Ser473.
Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase-silent mating type information regulation 2 homologue 1-PPARgamma co-activator-1alpha axis in rat mesangial cells under high-dose glucosamine.[Pubmed:25404010]
Br J Nutr. 2015 Jan 14;113(1):35-44.
Grape seed procyanidin B2 (GSPB2), an antioxidative and anti-inflammatory polyphenol in grape seed, has been found to have protective effects on diabetic nephropathy. Based on its favourable biological activities, in the present study, we aimed to investigate whether GSPB2 could inhibit apoptosis in rat mesangial cells treated with glucosamine (GlcN) under high-dose conditions. The results showed that the administration of GSPB2 (10 mug/ml) significantly increased the viability of mesangial cells treated with GlcN at a dose of 15 mM. We found that GSPB2 inhibited apoptosis in mesangial cells using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphates (dUTP) nick-end labelling staining and flow cytometry technique (P< 0.05 for both). GSPB2 treatment also suppressed oxidative stress by elevating the activity of glutathione peroxidase (P< 0.05) and superoxide dismutase (P< 0.01), as well as prevented cellular damage. GSPB2 enhanced the mRNA expression of nuclear respiratory factor 1, mitochondrial transcription factor A and mitochondrial DNA copy number in mesangial cells as determined by real-time PCR (P< 0.05 for each). Finally, GSPB2 treatment activated the protein expression of PPARgamma co-activator-1alpha (PGC-1alpha), silent mating type information regulation 2 homologue 1 (SIRT1) and AMP-activated protein kinase (AMPK) in mesangial cells. These findings suggest that GSPB2 markedly ameliorates mitochondrial dysfunction and inhibits apoptosis in rat mesangial cells treated with high-dose GlcN. This protective effect could be, at least in part, due to the activation of the AMPK-SIRT1-PGC-1alpha axis.
Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression.[Pubmed:19291307]
Neural Dev. 2009 Mar 16;4:11.
BACKGROUND: The homeodomain transcription factors Engrailed-1 and Engrailed-2 are required for the survival of mesencephalic dopaminergic (mesDA) neurons in a cell-autonomous and gene-dose-dependent manner. Homozygote mutant mice, deficient of both genes (En1-/-;En2-/-), die at birth and exhibit a loss of all mesDA neurons by mid-gestation. In heterozygote animals (En1+/-;En2-/-), which are viable and fertile, postnatal maintenance of the nigrostriatal dopaminergic system is afflicted, leading to a progressive degeneration specific to this subpopulation and Parkinson's disease-like molecular and behavioral deficits. RESULTS: In this work, we show that the dose of Engrailed is inversely correlated to the expression level of the pan-neurotrophin receptor gene P75NTR (Ngfr). Loss of mesDA neurons in the Engrailed-null mutant embryos is caused by elevated expression of this neurotrophin receptor: Unusually, in this case, the cell death signal of P75NTR is mediated by suppression of Erk1/2 (extracellular-signal-regulated kinase 1/2) activity. The reduction in expression of Engrailed, possibly related to the higher levels of P75NTR, also decreases mitochondrial stability. In particular, the dose of Engrailed determines the sensitivity to cell death induced by the classic Parkinson-model toxin MPTP and to inhibition of the anti-apoptotic members of the Bcl-2 family of proteins. CONCLUSION: Our study links the survival function of the Engrailed genes in developing mesDA neurons to the regulation of P75NTR and the sensitivity of these neurons to mitochondrial insult. The similarities to the disease etiology in combination with the nigral phenotype of En1+/-;En2-/- mice suggests that haplotype variations in the Engrailed genes and/or P75NTR that alter their expression levels could, in part, determine susceptibility to Parkinson's disease.
Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones.[Pubmed:19094096]
J Neuroendocrinol. 2009 Jan;21(1):77-81.
Recent findings indicate that progesterone can attenuate the beneficial neural effects of oestrogen. In the present study, we investigated the hypothesis that progesterone can modulate oestrogen actions by regulating the expression and activity of oestrogen receptors, ERalpha and ERbeta. Our studies in cultured neurones demonstrate that progesterone decreases the expression of both ERalpha and ERbeta and, as a consequence, also reduces both ER-dependent transcriptional activity and neuroprotection. These results identify a potential mechanism by which progesterone antagonises neural oestrogen actions, a finding that may have important implications for hormone therapy in postmenopausal women.


