CisplatinInhibits DNA synthesis,chemotherapy drug CAS# 14283-03-5 |
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Batimastat sodium salt
Catalog No.:BCC2075
CAS No.:130464-84-5
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- LY2334737
Catalog No.:BCC4060
CAS No.:892128-60-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14283-03-5 | SDF | Download SDF |
| PubChem ID | 84691 | Appearance | Powder |
| Formula | Cl2H6N2Pt | M.Wt | 300.05 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Synonyms | CDDP;Diamminedichloroplatinum | ||
| Solubility | Soluble to 5 mM in water with gentle warming | ||
| Chemical Name | cis-Diaminodichloroplatinum | ||
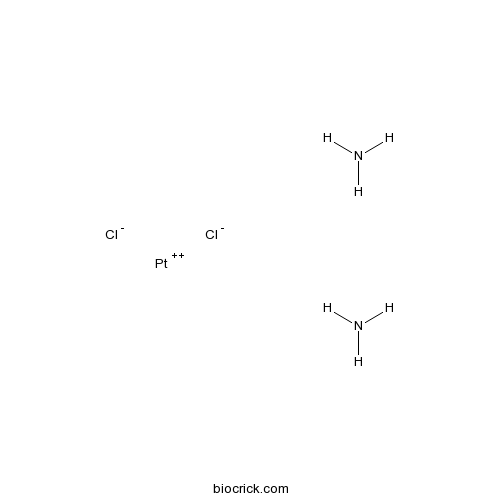
| SMILES | N.N.[Cl-].[Cl-].[Pt++] | ||
| Standard InChIKey | JFUARQHXOQHNLK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/2Cl.2H3N.Pt/h;;2*1H3; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cisplatin can enhance the cell-killing effect of radiation, an effect whose intensity varies with the schedule of administration. 2. The antitumor activity of the combination of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carhonyloxycamptothecin and CDDP(CPT-11) and cisplatin is superior to that of CPT-11 or cisplatin alone. 3. Cisplatin can induce acute kidney injury and neurotoxicity in rats. |

Cisplatin Dilution Calculator

Cisplatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3328 mL | 16.6639 mL | 33.3278 mL | 66.6556 mL | 83.3194 mL |
| 5 mM | 0.6666 mL | 3.3328 mL | 6.6656 mL | 13.3311 mL | 16.6639 mL |
| 10 mM | 0.3333 mL | 1.6664 mL | 3.3328 mL | 6.6656 mL | 8.3319 mL |
| 50 mM | 0.0667 mL | 0.3333 mL | 0.6666 mL | 1.3331 mL | 1.6664 mL |
| 100 mM | 0.0333 mL | 0.1666 mL | 0.3333 mL | 0.6666 mL | 0.8332 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cisplatin is a highly effective and broad-spectrum chemotherapeutic agent [1].
Cisplatin is an anticancer agent with some side effects. It is believed to induce apoptosis through several mechanisms. The traditional mechanism is that cisplatin enters the cell, interacts with the DNA guanine bases and forms the inter- or intra-strand chain cross-linking, then prevents the replication of DNA. This formation can also induce apoptosis by activating p53. Cisplatin was also found to cause ROS generation and increase lipid peroxidation, which leads cells to the apoptotic pathway. In addition, cisplatin induces apoptosis with the caspase-dependent pathway. In cochlear cells, cisplatin treatment results in the increase of caspases-3 and -9 and causes the cochlear damage side effect [1].
References:
[1] Casares C, Ramírez-Camacho R, Trinidad A, et al. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. European Archives of Oto-Rhino-Laryngology, 2012, 269(12): 2455-2459.
- Ryanodine
Catalog No.:BCC5742
CAS No.:15662-33-6
- 7,13-Dideacetyl-9,10-didebenzoyltaxchinin C
Catalog No.:BCN7670
CAS No.:156497-25-5
- 1-Hydroxytropacocaine
Catalog No.:BCN1919
CAS No.:156497-23-3
- Myricetin 3-O-galactoside
Catalog No.:BCN4703
CAS No.:15648-86-9
- α-Conotoxin ImI
Catalog No.:BCC5974
CAS No.:156467-85-5
- cis-Khellactone
Catalog No.:BCN3703
CAS No.:15645-11-1
- CWHM-12
Catalog No.:BCC5548
CAS No.:1564286-55-0
- 3,4-Seco-3-oxobisabol-10-ene-4,1-olide
Catalog No.:BCN7550
CAS No.:1564265-85-5
- Ailanthoidol
Catalog No.:BCN7705
CAS No.:156398-61-7
- Ehretioside B
Catalog No.:BCN1703
CAS No.:156368-84-2
- 11-Anhydro-16-oxoalisol A
Catalog No.:BCN7703
CAS No.:156338-93-1
- PyAOP
Catalog No.:BCC2819
CAS No.:156311-83-0
- 1,2,5-Trihydroxyxanthone
Catalog No.:BCN7585
CAS No.:156640-23-2
- Org 20599
Catalog No.:BCC7470
CAS No.:156685-94-8
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- SNC 80
Catalog No.:BCC6785
CAS No.:156727-74-1
- Delphinidin-3-O-rutinoside chloride
Catalog No.:BCN3115
CAS No.:15674-58-5
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
Regulation of renal organic anion and cation transporters by thymoquinone in cisplatin induced kidney injury.[Pubmed:22414646]
Food Chem Toxicol. 2012 May;50(5):1675-9.
In previous studies, we have demonstrated the biological activity of thymoquinone (TQ), an active compound extracted from the Nigella sativa plant, against Cisplatin-induced neurotoxicity. Recenty, it was observed that there is an inherent lack in regulation of renal organic anion and cation transporters in Cisplatin-induced nephrotoxicity. Here, we report, for the first time, the effect of TQ on alterations in the renal expression of organic anion transporters (OATs) and organic cation transporters (OCTs), as well as multidrug resistance-associated proteins (MRPs) in rats treated with Cisplatin. Twenty-eight 8-week-old male Wistar rats were divided into four groups of control, TQ treated (10 mg/kg b.w. in drinking water for 5 days), Cisplatin (7 mg/kg b.w., i.p.) and TQ and Cisplatin combination treatment. Cisplatin-induced malondialdehyde (MDA) and 8-isoprostane increase was found to be markedly reduced in rats treated with TQ. In Cisplatin only treated rats, the induced renal injury increased protein levels of the efflux transporters MRP2 and MRP4 while expression of OAT1, OAT3, OCT1 and OCT2 was reduced. In combination TQ- and Cisplatin-treated rats, expression of MRP2 and MRP4 proteins was decreased in the kidneys. Conversely, TQ treatment increased levels of OCT1, OCT2, OAT1 and OAT3 and decreased levels of 8-isoprostane and MDA levels in Cisplatin-treated rats. In conclusion, the present study shows that the TQ synergizes with its nephroprotective effect against Cisplatin-induced acute kidney injury in rats.
Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer.[Pubmed:1310160]
N Engl J Med. 1992 Feb 20;326(8):524-30.
BACKGROUND AND METHODS: Cisplatin (cis-diamminedichloroplatinum) has been reported to enhance the cell-killing effect of radiation, an effect whose intensity varies with the schedule of administration. We randomly assigned 331 patients with nonmetastatic inoperable non-small-cell lung cancer to one of three treatments: radiotherapy for two weeks (3 Gy given 10 times, in five fractions a week), followed by a three-week rest period and then radiotherapy for two more weeks (2.5 Gy given 10 times, five fractions a week); radiotherapy on the same schedule, combined with 30 mg of Cisplatin per square meter of body-surface area, given on the first day of each treatment week; or radiotherapy on the same schedule, combined with 6 mg of Cisplatin per square meter, given daily before radiotherapy. RESULTS: Survival was significantly improved in the radiotherapy-daily-Cisplatin group as compared with the radiotherapy group (P = 0.009): survival in the radiotherapy-daily-Cisplatin group was 54 percent at one year, 26 percent at two years, and 16 percent at three years, as compared with 46 percent, 13 percent, and 2 percent, respectively, in the radiotherapy group. Survival in the radiotherapy-weekly-Cisplatin group was intermediate (44 percent, 19 percent, and 13 percent) and not significantly different from survival in either of the other two groups. The survival benefit of daily combined treatment was due to improved control of local disease (P = 0.003). Survival without local recurrence was 59 percent at one year and 31 percent at two years in the radiotherapy-daily-Cisplatin group; 42 percent and 30 percent, respectively, in the radiotherapy-weekly-Cisplatin group; and 41 percent and 19 percent, respectively, in the radiotherapy group. Cisplatin induced nausea and vomiting in 86 percent of the patients given it weekly and in 78 percent of those given it daily; these effects were severe in 26 percent and 28 percent, respectively. CONCLUSIONS: Cisplatin, given daily in combination with the radiotherapy described here to patients with nonmetastatic but inoperable non-small-cell lung cancer, improved rates of survival and control of local disease at the price of substantial side effects.
Enhanced antitumor efficacy of a combination of CPT-11, a new derivative of camptothecin, and cisplatin against human lung tumor xenografts.[Pubmed:8385085]
Jpn J Cancer Res. 1993 Feb;84(2):203-7.
The objective of this study was to evaluate the antitumor efficacy of combined use of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11) and Cisplatin (CDDP). The antitumor activities of CPT-11, CDDP and their combination against 3 human lung tumor xenografts were estimated using congenitally athymic BALB/c (nu/nu) mice. The doses were 47 mg/kg for CPT-11 and 6 mg/kg for CDDP on days 1, 5 and 9. In combination therapy, half of the single dosage of each agent was used. The doses were administered intraperitoneally. The antitumor activity and toxicity were evaluated in terms of the tumor volume and body weight change of mice, respectively. The combination therapy resulted in a statistically significant tumor regression compared to the use of only CPT-11 or CDDP in two tumor xenografts out of three. The toxicity of the combination therapy was no higher than that of CPT-11 or CDDP alone. These results suggest that the antitumor activity of the combination of CPT-11 and CDDP is superior to that of CPT-11 or CDDP alone.


