Org 20599Positive allosteric modulator and direct agonist of GABAA receptors CAS# 156685-94-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156685-94-8 | SDF | Download SDF |
| PubChem ID | 9850026 | Appearance | Powder |
| Formula | C25H40ClNO3 | M.Wt | 438.04 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl and to 100 mM in DMSO | ||
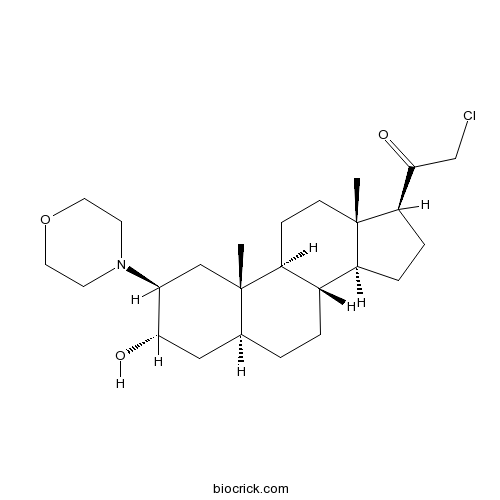
| Chemical Name | 2-chloro-1-[(2S,3S,5S,8R,9S,10S,13S,14S,17S)-3-hydroxy-10,13-dimethyl-2-morpholin-4-yl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]ethanone | ||
| SMILES | CC12CCC3C(C1CCC2C(=O)CCl)CCC4C3(CC(C(C4)O)N5CCOCC5)C | ||
| Standard InChIKey | NZFNABGZEQPYBX-PMBZPZLSSA-N | ||
| Standard InChI | InChI=1S/C25H40ClNO3/c1-24-8-7-19-17(18(24)5-6-20(24)23(29)15-26)4-3-16-13-22(28)21(14-25(16,19)2)27-9-11-30-12-10-27/h16-22,28H,3-15H2,1-2H3/t16-,17-,18-,19-,20+,21-,22-,24-,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Positive allosteric modulator and at higher concentrations direct agonist of GABAA receptors (EC50 = 1.1 μM). Also displays positive modulation of glycine receptors (EC50 = 22.9 μM). Anesthetic steroid; induces hypnosis and loss of righting reflexes in mice. |

Org 20599 Dilution Calculator

Org 20599 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2829 mL | 11.4145 mL | 22.829 mL | 45.6579 mL | 57.0724 mL |
| 5 mM | 0.4566 mL | 2.2829 mL | 4.5658 mL | 9.1316 mL | 11.4145 mL |
| 10 mM | 0.2283 mL | 1.1414 mL | 2.2829 mL | 4.5658 mL | 5.7072 mL |
| 50 mM | 0.0457 mL | 0.2283 mL | 0.4566 mL | 0.9132 mL | 1.1414 mL |
| 100 mM | 0.0228 mL | 0.1141 mL | 0.2283 mL | 0.4566 mL | 0.5707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,2,5-Trihydroxyxanthone
Catalog No.:BCN7585
CAS No.:156640-23-2
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Ryanodine
Catalog No.:BCC5742
CAS No.:15662-33-6
- 7,13-Dideacetyl-9,10-didebenzoyltaxchinin C
Catalog No.:BCN7670
CAS No.:156497-25-5
- 1-Hydroxytropacocaine
Catalog No.:BCN1919
CAS No.:156497-23-3
- Myricetin 3-O-galactoside
Catalog No.:BCN4703
CAS No.:15648-86-9
- α-Conotoxin ImI
Catalog No.:BCC5974
CAS No.:156467-85-5
- cis-Khellactone
Catalog No.:BCN3703
CAS No.:15645-11-1
- CWHM-12
Catalog No.:BCC5548
CAS No.:1564286-55-0
- 3,4-Seco-3-oxobisabol-10-ene-4,1-olide
Catalog No.:BCN7550
CAS No.:1564265-85-5
- Ailanthoidol
Catalog No.:BCN7705
CAS No.:156398-61-7
- Ehretioside B
Catalog No.:BCN1703
CAS No.:156368-84-2
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- SNC 80
Catalog No.:BCC6785
CAS No.:156727-74-1
- Delphinidin-3-O-rutinoside chloride
Catalog No.:BCN3115
CAS No.:15674-58-5
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
- Cephalexin
Catalog No.:BCC4646
CAS No.:15686-71-2
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
The anaesthetic action and modulation of GABAA receptor activity by the novel water-soluble aminosteroid Org 20599.[Pubmed:9014136]
Neuropharmacology. 1996;35(9-10):1209-22.
The anaesthetic profile of a novel water-soluble aminosteroid, Org 20599 [(2 beta, 3 alpha, 5 alpha)-21-chloro-3-hydroxy-2-(4-morpholinyl)pregnan-20-one methanesulphonate], and the ability of the compound to allosterically regulate the activity of the GABAA receptor, have been studied in comparison to the properties of established intravenous general-anaesthetic agents. Intravenously administered Org 20599 produced a rapid onset, short duration loss of the righting reflex in mice. The anaesthetic potency of Org 20599 was comparable to that of the steroids 5 alpha-pregnan-3 alpha-ol-20-one or alphaxalone, and exceeded that of propofol, thiopentone or pentobarbitone. Org 20599 and the reference anaesthetic agents allosterically displaced the binding of [35S]-t-butylbicyclophosphorothionate (TBPS) from GABAA receptors of rat-brain membranes with the order of potency: 5 alpha-pregnan-3 alpha-ol-20-one > Org 20599 > alphaxalone > propofol > thiopentone > pentobarbitone. At human recombinant alpha 1, beta 2, gamma 2L subunit-containing GABAA receptors expressed in Xenopus laevis oocytes, the anaesthetic agents produced a concentration-dependent and reversible potentiation of the peak amplitude of GABA-evoked currents. A similar positive allosteric action of Org 20599 was observed for the GABAA receptors expressed by bovine adrenal chromaffin cells maintained in culture. The rank order of potency in the aforementioned assays was identical to that determined from the displacement of TBPS binding. At concentrations greater than those required for potentiation of GABA, the anaesthetics exhibited GABA-mimetic effects with a rank order of potency that paralleled their modulatory activity. Such direct agonism varied greatly in maximal effect between compounds. The modulatory and direct agonist actions of Org 20599 were additionally confirmed utilizing rat hippocampal neurones in culture. The results indicate Org 20599 to be a potent and short-acting intravenous anaesthetic agent in mice and suggest positive allosteric regulation of GABAA receptor function to be a plausible molecular mechanism of action for the drug.
The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors.[Pubmed:15033889]
Br J Anaesth. 2004 May;92(5):704-11.
BACKGROUND: Anaesthetic steroids are established positive allosteric modulators of GABAA receptors, but little is known concerning steroid modulation of strychnine-sensitive glycine receptors, the principal mediators of fast, inhibitory neurotransmission in the brain stem and spinal cord. This study compared the modulatory actions of five anaesthetic pregnane steroids and two non-anaesthetic isomers at human recombinant alpha1 glycine and alpha1beta2gamma2L GABAA receptors. METHODS: Recombinant alpha1 glycine or alpha1beta2gamma2L GABAA receptors were expressed in Xenopus laevis oocytes and agonist-evoked currents recorded under voltage-clamp. Steroid modulation of currents evoked by GABA, or glycine, was quantified by determining the potency (EC50) and maximal effect of the compounds. RESULTS: The anaesthetics minaxolone (EC50=1.3 microM), Org20599 (EC50=1.1 microM) and alphaxalone (EC50=2.2 microM) enhanced currents mediated by GABAA receptors. The anaesthetics also enhanced currents mediated by glycine receptors, although with higher EC50 values (minaxolone 13.1 microM; Org20599=22.9 microM and alphaxalone=27.8 microM). The maximal enhancement (to 780-950% of control) produced by the three steroids acting at the GABAA receptor was similar, but currents evoked by glycine were potentiated with increasing effectiveness by alphaxalone (199%) The objective of the present investigation was to characterize the in vivo EEG effects of (synthetic) neuroactive steroids on the basis of a recently proposed mechanism-based pharmacokinetic/pharmacodynamic (PK/PD) model. After intravenous administration, the time course of the EEG effect of pregnanolone, 2beta-3alpha-5alpha-3-hydroxy-2-(2,2-dimethylmorpholin-4-yl)-pregnan-11,20-dione (ORG 21465), 2beta-3alpha-5alpha-21-chloro-3-hydroxy-2-(4-morpholinyl)-pregnan-20-one (Org 20599), and alphaxalone was determined in conjunction with plasma concentrations in rats. For each neuroactive steroid the PK/PD correlation was described on the basis of a two-compartment pharmacokinetic model with an effect compartment to account for hysteresis. The observed concentration EEG effect relationships were biphasic and characterized with a mechanism-based pharmacodynamic model, which is based on a separation between the receptor activation process and the stimulus-response relationship. A single unique biphasic stimulus-response relationship could be identified for all neuroactive steroids, which was successfully described by a parabolic function. The receptor activation process was described by a hyperbolic function. Estimates for the maximum activation (e(PD)) were similar for the different neuroactive steroids but values of the potency estimate (K(PD)) ranged from 157 +/- 16 ng. ml(-1) for pregnanolone, 221 +/- 83 ng. ml(-1) for Org 20599, and 483 +/- 42 ng. ml(-1) for alphaxalone to 1619 +/- 208 ng. ml(-1) for ORG 21465. A statistically significant correlation was observed between the in vivo potency and the IC(50) in an in vitro [(35)S]t-butylbicyclophosphorothionate binding assay (r = 0.91). It is concluded that the new PK/PD model constitutes a new mechanism-based approach to the quantification of the effects of (synthetic) neuroactive steroids in vivo effects. The results show that the neuroactive steroids differ in potency but not in intrinsic efficacy at the GABA(A) receptor in vivo.Neuroactive steroids differ in potency but not in intrinsic efficacy at the GABA(A) receptor in vivo.[Pubmed:12388643]
J Pharmacol Exp Ther. 2002 Nov;303(2):616-26.


