SNC 80CAS# 156727-74-1 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156727-74-1 | SDF | Download SDF |
| PubChem ID | 123924 | Appearance | Powder |
| Formula | C28H39N3O2 | M.Wt | 449.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl with gentle warming and to 20 mM in DMSO | ||
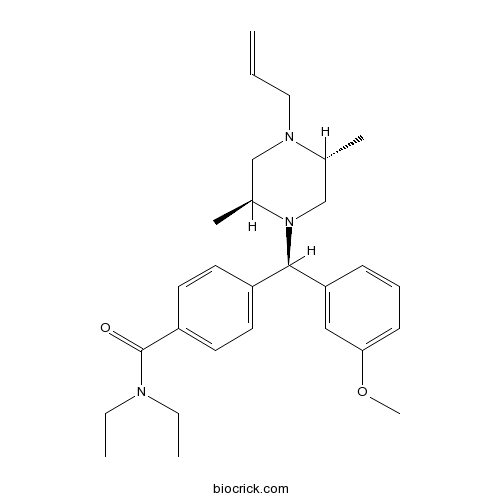
| Chemical Name | 4-[(R)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide | ||
| SMILES | CCN(CC)C(=O)C1=CC=C(C=C1)C(C2=CC(=CC=C2)OC)N3CC(N(CC3C)CC=C)C | ||
| Standard InChIKey | KQWVAUSXZDRQPZ-UMTXDNHDSA-N | ||
| Standard InChI | InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A highly selective and potent non-peptide δ-opioid agonist, 2000-fold selective over μ-opioid receptors. |

SNC 80 Dilution Calculator

SNC 80 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.224 mL | 11.12 mL | 22.24 mL | 44.48 mL | 55.6 mL |
| 5 mM | 0.4448 mL | 2.224 mL | 4.448 mL | 8.896 mL | 11.12 mL |
| 10 mM | 0.2224 mL | 1.112 mL | 2.224 mL | 4.448 mL | 5.56 mL |
| 50 mM | 0.0445 mL | 0.2224 mL | 0.4448 mL | 0.8896 mL | 1.112 mL |
| 100 mM | 0.0222 mL | 0.1112 mL | 0.2224 mL | 0.4448 mL | 0.556 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- Org 20599
Catalog No.:BCC7470
CAS No.:156685-94-8
- 1,2,5-Trihydroxyxanthone
Catalog No.:BCN7585
CAS No.:156640-23-2
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Ryanodine
Catalog No.:BCC5742
CAS No.:15662-33-6
- 7,13-Dideacetyl-9,10-didebenzoyltaxchinin C
Catalog No.:BCN7670
CAS No.:156497-25-5
- 1-Hydroxytropacocaine
Catalog No.:BCN1919
CAS No.:156497-23-3
- Myricetin 3-O-galactoside
Catalog No.:BCN4703
CAS No.:15648-86-9
- α-Conotoxin ImI
Catalog No.:BCC5974
CAS No.:156467-85-5
- cis-Khellactone
Catalog No.:BCN3703
CAS No.:15645-11-1
- CWHM-12
Catalog No.:BCC5548
CAS No.:1564286-55-0
- 3,4-Seco-3-oxobisabol-10-ene-4,1-olide
Catalog No.:BCN7550
CAS No.:1564265-85-5
- Delphinidin-3-O-rutinoside chloride
Catalog No.:BCN3115
CAS No.:15674-58-5
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
- Cephalexin
Catalog No.:BCC4646
CAS No.:15686-71-2
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
- N1,N11-Diethylnorspermine tetrahydrochloride
Catalog No.:BCC6654
CAS No.:156886-85-0
- Licofelone
Catalog No.:BCC4432
CAS No.:156897-06-2
Brain delta2 opioid receptors mediate SNC-80-evoked hypothermia in rats.[Pubmed:15936000]
Brain Res. 2005 Jul 5;1049(1):61-9.
Despite insights into an increasingly significant role for delta opioid receptors in thermoregulation, it is unclear whether delta receptors located in the brain or periphery play the more critical role in body temperature regulation. Moreover, it is not entirely clear which delta receptor phenotype, delta1 or delta2, mediates the hypothermic actions of delta agonists. Because SNC-80 distributes into central and peripheral compartments and produces rapid hypothermia following systemic injection, the nonpeptide delta agonist is particularly useful in discriminating the site of action of delta receptor-mediated hypothermia. To determine the locus and phenotype of delta receptor which mediates SNC-80-induced hypothermia, we injected SNC-80 and phenotype selective delta antagonists to male Sprague-Dawley rats. SNC-80 (10-50 mg/kg, im) evoked hypothermia that peaked 30 min post-injection. Naltrexone (5 mg/kg, sc), an opioid antagonist, or naltrindole (5 mg/kg, sc), a delta antagonist, blocked the hypothermic response to SNC-80 (35 mg/kg, im). The hypothermia caused by SNC-80 (35 mg/kg, im) was blocked by a delta2 antagonist, naltriben (2.5 mg/kg, sc), but was not affected by BNTX (5 and 10 mg/kg, sc), a delta1 antagonist. The administration of naltriben (10 microg/rat, icv) 30 min before SNC-80 (35 mg/kg, im) prevented SNC-80-evoked hypothermia. In contrast, methylnaltrexone (5 mg/kg, sc), a peripherally restricted opioid antagonist, did not affect the hypothermia caused by SNC-80. The present data demonstrate that selective activation of brain delta2 receptors is a major mechanism of SNC-80-evoked hypothermia in rats.
Chemotaxis of human and rat leukocytes by the delta-selective non-peptidic opioid SNC 80.[Pubmed:17061517]
Rev Latinoam Microbiol. 2003 Jan-Jun;45(1-2):16-23.
Opioids like morphine, represent a major source of relief for most chronic moderate to severe nonmalignant pain. However, opioid abuse may lead to infections such as hepatitis and AIDS because opioids have been associated with suppressing various parameters of immune function including antimicrobial resistance, antibody production, monocyte-mediated phagocytosis, and both neutrophil and monocyte chemotaxis. We have previously reported immunopotentiating properties of non-peptidic opioid receptor selective agonists and antagonists. In this study, we evaluated the effects of the nonpeptidic delta-opioid receptor agonist (+)-4-((alpha R)-alpha-((2S, 5R)-4-allyl-2, 5-dimethyl-1-piperazinyl)-3-methoxybenzyl)-N, N-diethyl-benzamide (SNC 80) on chemotaxis of rat thymic and human peripheral blood mononuclear cells by using a modified Wilkinson chamber. Cell recruitment is an essential process in acute and chronic inflammatory responses. We observed that SNC 80 at concentrations of 10(-10), 10(-9), 10(-8), 10(-7), and 10(-6) M, significantly (p < 0.01) stimulated rat thymic (1.3, 1.55, 1.58, 1.75, and 1.8-fold increases respectively) and human leukocyte (1.13, 1.37, 1.43, 1.7, 1.83 fold-increases respectively) chemotaxis (demonstrated by checkerboard assays), compared with untreated control. The effects of SNC 80 on chemotaxis of rat and human leukocytes were antagonized by naloxone, indicating that the modulation of chemotaxis by SNC 80 is via a classic opioid receptor. The development and use of non-peptidic opioids like SNC 80 could have an immediate impact not only as potent analgesics, but in immunoregulation.
Molecular modeling directed synthesis of a bicyclic analogue of the delta opioid receptor agonist SNC 80.[Pubmed:15938001]
Arch Pharm (Weinheim). 2005 Jun;338(5-6):281-90.
In order to find novel delta opioid receptor agonists, the pharmacophoric benzhydryl moiety of the lead compound SNC 80 (1) was dissected and the phenyl residues were attached to different positions of the 6,8-diazabicyclo[3.2.2]nonane core system (4). The position of the carboxamido group, the stereochemistry, the C3/C4 bond order and the kind and length of the spacer X were considered. The resulting compounds were compared with the four energetically most favourable conformations of SNC 80 by a multifit analysis. These calculations led to the structures 5-10, which fit best to SNC 80. Herein the synthesis of one of these compounds (9) is described. Starting from (S)-glutamate two alternative routes are detailed to obtain the key intermediate 14. A variation of the Dieckmann cyclization, which uses trapping of the first cyclization product with ClSiMe(3) provided the mixed acetal 20, which was carefully hydrolyzed to yield the bicyclic ketone 17. Stereoselective addition of phenylmagnesium bromide, dehydration, LiAlH(4) reduction and exchange of the N-6 residue afforded the designed compound 9. The affinities of 9 towards delta, mu, kappa and ORL1 receptors were determined in receptor binding studies with radioligands. Only moderate receptor affinity was found.
Increased survival of tumor-bearing mice by the delta opioid SNC 80.[Pubmed:16334142]
Anticancer Res. 2005 Nov-Dec;25(6C):4563-7.
Opioids represent a major source of relief from pain. However, opioid abuse may cause immunosuppression and cancer. We have recently reported results on novel non-peptidic delta- and mu-selective opioids that induced immunopotentiation of T cell and macrophage functions in vitro and ex vivo. In the present study, the effects of the delta-opioid receptor agonist and potent analgesic (+)-4-((alpha R)-alpha-((2S, 5R)-4-allyl-2, 5-dimethyl-1-piperazinyl)-3-methoxybenzyl)-N, N-diethyl-benzamide (SNC80) on in vitro and in vivo tumor cell growth were investigated using the L5178Y-R murine model. SNC80 marginally, but significantly (p < 0.05), inhibited (up to 14%) the in vitro growth of L5178Y-R tumor cells. However, in vivo intratumor administration of SNC80 (2 and 4 mg/kg) reduced up to 60% L5178Y-R tumor-bearing Balb/c mice death, and significantly (p < 0.05) reduced tumor weights (up to 73% reduction) in these animals. This study may support the evaluation of SNC80 in preclinical and clinical studies.
Structure-activity relationships for SNC80 and related compounds at cloned human delta and mu opioid receptors.[Pubmed:8667189]
J Pharmacol Exp Ther. 1996 Jun;277(3):1284-91.
The racemic compound (+/-)-BW373U86 inverted question mark(+/-)-4-((alpha R*)- alpha-((2S*,5R*)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxy- benzyl)-N,N-diethylbenzamide dihydrochloride} is a potent delta opioid receptor agonist in the mouse vas deferens assay with little mu or kappa opioid receptor activity in the guinea pig ileum tissue preparation. In contrast, radioligand binding studies show that (+/-)-BW373U86 is only about 10-fold selective for delta over mu opioid receptors. Studies of the enantiomeric forms of (+/-)-BW373U86 and derivatives (SNC80 and related compounds) show that some of these isomers are significantly better in both receptor binding and pharmacological selectivity than (+/-)-BW373U86. In this study we have determined the binding affinities of 10 different SNC80-related compounds at cloned human delta and mu opioid receptors and measured the potency of SNC80 for the inhibition of forskolin-stimulated adenylyl cyclase. The most selective delta receptor ligand (SNC162) differed from SNC80 by the absence of the 3-methoxy substitution of the benzyl ring. The Ki for SNC162 at the delta receptor (0.625 nM) was over 8700-fold lower than that at the mu receptor (5500 nM), making this the most selective delta receptor ligand available. Reduction of the allyl side chain of SNC80 to produce radiolabeled [3H]SNC121 allowed direct measurement of the association and dissociation rate constants. SNC80 was 26-fold less potent than [D-Pen2, pCI-Phe4, D-Pen5]enkephalin in the delta receptor adenylyl cyclase inhibition assay, but showed full agonist activity with an EC50 value of 9.2 nM. The regulation of SNC80 binding affinity to the delta receptor by GTP analogs is undetectable in [3H]naltrindole binding inhibition studies, but direct binding studies with [3H]SNC121 in the presence of 100 microM 5'-guanylylimidotriphosphate show a 55% reduction in maximum binding site density consistent with a lower affinity for a part of the receptor population. Addition of 120 mM sodium chloride reduces SNC80 affinity nearly 40-fold in [3H]naltrindole binding inhibition studies. The results of these studies define specific structural features of these compounds responsible for opioid receptor interactions and suggest a possibly novel mechanism for delta receptor activation.
SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist.[Pubmed:7714789]
J Pharmacol Exp Ther. 1995 Apr;273(1):359-66.
The present study has investigated the pharmacology of SNC 80, a nonpeptidic ligand proposed to be a selective delta agonist in vitro and in vivo. SNC 80 was potent in producing inhibition of electrically induced contractions of mouse vas deferens, but not in inhibiting contractions of the guinea pig isolated ileum (IC50 values of 2.73 nM and 5457 nM, respectively). The delta selective antagonist ICI 174,864 (1 microM) and the mu selective antagonist CTAP (1 microM) produced 236- and 1.9-fold increases, respectively, in the SNC 80 IC50 value in the mouse vas deferens. SNC 80 preferentially competed against sites labeled by [3H]naltrindole (delta receptors) rather than against those labeled by [3H]DAMGO (mu receptors) or [3H]U69, 593 kappa receptors) in mouse whole-brain assays. The ratios of the calculated Ki values for SNC 80 at mu/delta and kappa/delta sites were 495- and 248-fold, respectively, which indicates a significant degree of delta selectivity for this compound in radioligand binding assays. SNC 80 produced dose- and time-related antinociception in the mouse warm-water tail-flick test after i.c.v., i.th. and i.p. administration. The calculated A50 values (and 95% C.I.) for SNC 80 administered i.c.v., i.th. and i.p. were 104.9 (63.7-172.7) nmol, 69 (51.8-92.1) nmol and 57 (44.5-73.1) mg/kg, respectively. The i.c.v. administration of SNC 80 also produced dose- and time-related antinociception in the hot-plate test, with a calculated A50 value (and 95% C.I.) of 91.9 (60.3-140.0) nmol.(ABSTRACT TRUNCATED AT 250 WORDS)


