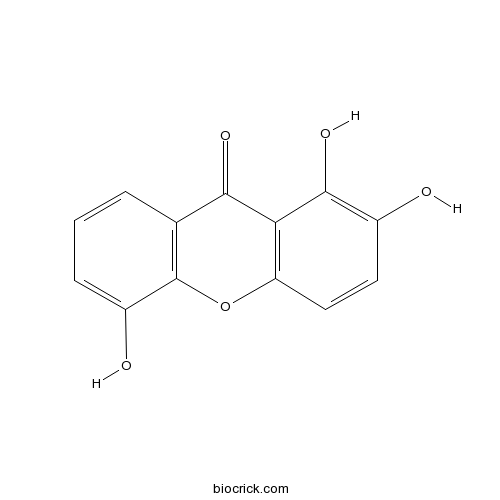
1,2,5-TrihydroxyxanthoneCAS# 156640-23-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156640-23-2 | SDF | Download SDF |
| PubChem ID | 10060362 | Appearance | Powder |
| Formula | C13H8O5 | M.Wt | 244.20 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2,5-trihydroxyxanthen-9-one | ||
| SMILES | C1=CC2=C(C(=C1)O)OC3=C(C2=O)C(=C(C=C3)O)O | ||
| Standard InChIKey | WRYMABXVDKBFIK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8O5/c14-7-4-5-9-10(12(7)17)11(16)6-2-1-3-8(15)13(6)18-9/h1-5,14-15,17H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 1,2,5-Trihydroxyxanthone has antioxidative properties. |

1,2,5-Trihydroxyxanthone Dilution Calculator

1,2,5-Trihydroxyxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.095 mL | 20.475 mL | 40.95 mL | 81.9001 mL | 102.3751 mL |
| 5 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 10 mM | 0.4095 mL | 2.0475 mL | 4.095 mL | 8.19 mL | 10.2375 mL |
| 50 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| 100 mM | 0.041 mL | 0.2048 mL | 0.4095 mL | 0.819 mL | 1.0238 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Ryanodine
Catalog No.:BCC5742
CAS No.:15662-33-6
- 7,13-Dideacetyl-9,10-didebenzoyltaxchinin C
Catalog No.:BCN7670
CAS No.:156497-25-5
- 1-Hydroxytropacocaine
Catalog No.:BCN1919
CAS No.:156497-23-3
- Myricetin 3-O-galactoside
Catalog No.:BCN4703
CAS No.:15648-86-9
- α-Conotoxin ImI
Catalog No.:BCC5974
CAS No.:156467-85-5
- cis-Khellactone
Catalog No.:BCN3703
CAS No.:15645-11-1
- CWHM-12
Catalog No.:BCC5548
CAS No.:1564286-55-0
- 3,4-Seco-3-oxobisabol-10-ene-4,1-olide
Catalog No.:BCN7550
CAS No.:1564265-85-5
- Ailanthoidol
Catalog No.:BCN7705
CAS No.:156398-61-7
- Ehretioside B
Catalog No.:BCN1703
CAS No.:156368-84-2
- 11-Anhydro-16-oxoalisol A
Catalog No.:BCN7703
CAS No.:156338-93-1
- Org 20599
Catalog No.:BCC7470
CAS No.:156685-94-8
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- SNC 80
Catalog No.:BCC6785
CAS No.:156727-74-1
- Delphinidin-3-O-rutinoside chloride
Catalog No.:BCN3115
CAS No.:15674-58-5
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
- Cephalexin
Catalog No.:BCC4646
CAS No.:15686-71-2
Xanthones from Hypericum japonicum and H. henryi.[Pubmed:9842731]
Phytochemistry. 1998 Nov;49(5):1395-402.
From the aerial part of Hypericum japonicum, one new xanthone glycoside, 1,5-dihydroxyxanthone-6-O-beta-D-glucoside, one novel dimer xanthone, bijaponicaxanthone, and the first natural prenylated xanthone, 1,3,5,6-tetrahydroxy-4-prenylxanthone, were isolated together with the four known xanthones, 1,5,6-trihydroxyxanthone, isojacereubin, 6-deoxyisojacareubin and 4',5'-dihydro-1,5,6-trihydroxy-4',4',5'-trimethylfurano (2',3':4,5) xanthone. five previously known xanthones, kielcorin, cadensin, 1,7,-dihydroxyxanthone, 1,5-dihydroxy-4-methoxyxanthone and 1,2,5-Trihydroxyxanthone were also found in the dichoromethane extract of the stems and leaves of H. henryi. Their structures were elucidated by spectroscopic and chemical methods. Some of the compounds from H. japonicum were found to exert an interesting coagulant activity in an in vitro test. The chemotaxonomic value of xanthones is discussed briefly.
[Chemical constituents from barks of Garcinia tetralata].[Pubmed:19157125]
Zhongguo Zhong Yao Za Zhi. 2008 Oct;33(20):2350-2.
OBJECTIVE: To study the chemical constituents of the barks of Garcinia tetralata. METHOD: Compounds were isolated and purified repeatedly by silica gel and ODS column chromatography and their chemical structures were elucidated by their physicochemical properties and spectral data analysis. RESULT: Ten compounds were obtained and identified as 1, 7-dihydroxyxanthone (1), buchanaxanthone (2), garciniaxanthone H (3), 1, 2, 5-trihydroxyxanthone (4), subelliptenone H (5), 6-desoxyjacareubin (6), 6-deoxyisojacreubin (7), subelliptenone G (8), methyl orsellinate (9) and beta-sitosterol (10). CONCLUSION: Compounds 1-10 were isolated from this plant and compound 9 was obtained from the genus Garcinia for the first time.


