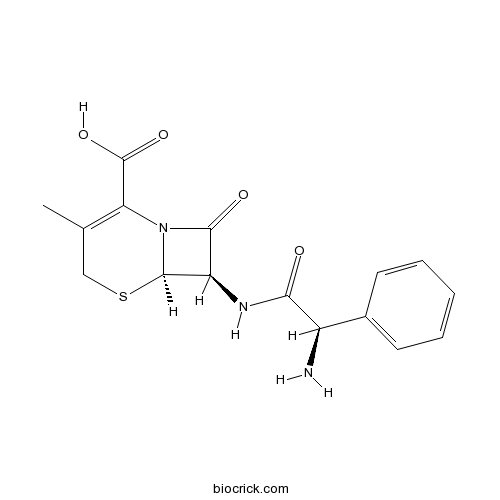
CephalexinCAS# 15686-71-2 |
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- XL019
Catalog No.:BCC2057
CAS No.:945755-56-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15686-71-2 | SDF | Download SDF |
| PubChem ID | 27447 | Appearance | Powder |
| Formula | C16H17N3O4S | M.Wt | 347.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Cefalexin; Cephacillin | ||
| Solubility | H2O : 10 mg/mL (28.79 mM; Need ultrasonic and warming) | ||
| Chemical Name | (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | ||
| SMILES | CC1=C(N2C(C(C2=O)NC(=O)C(C3=CC=CC=C3)N)SC1)C(=O)O | ||
| Standard InChIKey | ZAIPMKNFIOOWCQ-UEKVPHQBSA-N | ||
| Standard InChI | InChI=1S/C16H17N3O4S/c1-8-7-24-15-11(14(21)19(15)12(8)16(22)23)18-13(20)10(17)9-5-3-2-4-6-9/h2-6,10-11,15H,7,17H2,1H3,(H,18,20)(H,22,23)/t10-,11-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cefalexin is a cephalosporin antibiotic.
Target: Antibacterial
Cefalexin (INN, BAN) or cephalexin (USAN, AAN) is a first-generation cephalosporin antibiotic introduced in 1967 by Eli Lilly and Company. It is an orally administered agent with a similar antimicrobial spectrum to the intravenous agents cefalotin and cefazolin. It was first marketed as Keflex (Lilly), and is marketed under several other trade names. As of 2008, cefalexin was the most popular cephalosporin antibiotic in the United States, with more than 25 million prescriptions of its generic versions alone, for US$255 million in sales (though less popular than two other antibiotics, amoxicillin and azithromycin, each with 50 million prescriptions per year).
Cefalexin is marketed by generic pharmaceutical manufacturers under a wide range of brand names, including: Apo-Cephalex, Biocef, Cefanox, Ceforal, Cephabos, Cephalexin, Cephorum, Ceporex, Cilex, Ialex, Ibilex, Kefexin, Keflet, Keflex, Rekosporin, Keforal, Keftab, Keftal, Lopilexin, Larixin, Novo-Lexin, Ospexin, Tenkorex, Zephalexin, Panixine Disperdose, Rancef, Sialexin, Sporidex and Ulexin. A version of Keflex 750 mg capsules is marketed for twice-daily dosage, to improve compliance. However, it is not a sustained release formulation, and since it is more expensive than the older strengths, some physicians prescribe three 250 mg capsules to be taken twice daily, as a less expensive alternative. References: | |||||

Cephalexin Dilution Calculator

Cephalexin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8786 mL | 14.393 mL | 28.7861 mL | 57.5722 mL | 71.9652 mL |
| 5 mM | 0.5757 mL | 2.8786 mL | 5.7572 mL | 11.5144 mL | 14.393 mL |
| 10 mM | 0.2879 mL | 1.4393 mL | 2.8786 mL | 5.7572 mL | 7.1965 mL |
| 50 mM | 0.0576 mL | 0.2879 mL | 0.5757 mL | 1.1514 mL | 1.4393 mL |
| 100 mM | 0.0288 mL | 0.1439 mL | 0.2879 mL | 0.5757 mL | 0.7197 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cefalexin is a cephalosporin antibiotic.
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Delphinidin-3-O-rutinoside chloride
Catalog No.:BCN3115
CAS No.:15674-58-5
- SNC 80
Catalog No.:BCC6785
CAS No.:156727-74-1
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- Org 20599
Catalog No.:BCC7470
CAS No.:156685-94-8
- 1,2,5-Trihydroxyxanthone
Catalog No.:BCN7585
CAS No.:156640-23-2
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
- N1,N11-Diethylnorspermine tetrahydrochloride
Catalog No.:BCC6654
CAS No.:156886-85-0
- Licofelone
Catalog No.:BCC4432
CAS No.:156897-06-2
- OSU 6162 hydrochloride
Catalog No.:BCC7424
CAS No.:156907-84-5
- Isosalicifolin
Catalog No.:BCN6502
CAS No.:156974-99-1
- Grosvenorin
Catalog No.:BCN1262
CAS No.:156980-60-8
- Wedeliatrilolactone A
Catalog No.:BCN6732
CAS No.:156993-29-2
- H-D-Arg-OH
Catalog No.:BCC2868
CAS No.:157-06-2
- 5,7-Dihydroxy-3,4',8-trimethoxyflavone
Catalog No.:BCN1551
CAS No.:1570-09-8
- U 89843A
Catalog No.:BCC7466
CAS No.:157013-85-9
- SNAP 5089
Catalog No.:BCC7350
CAS No.:157066-77-8
- Lipedoside B-III
Catalog No.:BCC8201
CAS No.:157085-48-8
Electrochemical mineralization of cephalexin using a conductive diamond anode: A mechanistic and toxicity investigation.[Pubmed:27847122]
Chemosphere. 2017 Feb;168:638-647.
The contamination of surface and ground water by antibiotics is of significant importance due to their potential chronic toxic effects to the aquatic and human lives. Thus, in this work, the electrochemical oxidation of Cephalexin (CEX) was carried out in a one compartment filter-press flow cell using a boron-doped diamond (BDD) electrode as anode. During the electrolysis, the investigated variables were: supporting electrolyte (Na2SO4, NaCl, NaNO3, and Na2CO3) at constant ionic strength (0.1 M), pH (3, 7, 10, and without control), and current density (5, 10 and 20 mA cm(-2)). The oxidation and mineralization of CEX were assessed by high performance liquid chromatography, coupled to mass spectrometry and total organic carbon. The oxidation process of CEX was dependent on the type of electrolyte and on pH of the solution due to the distinct oxidant species electrogenerated; however, the conversion of CEX and its hydroxylated intermediates to CO2 depends only on their diffusion to the surface of the BDD. In the final stages of electrolysis, an accumulation of recalcitrant oxamic and oxalic carboxylic acids, was detected. Finally, the growth inhibition assay with Escherichia coli cells showed that the toxicity of CEX solution decreased along the electrochemical treatment due to the rupture of the beta-lactam ring of the antibiotic.
Delayed Anaphylaxis with Methimazole: Nicolau Syndrome After Oxytocin Intramuscular Administration Anastrazole-Induced Autoimmune Hepatitis Amoxicillin- and Cephalexin-Induced Eosinophilic Colitis Docetaxel-Induced Supravenous Erythematous Eruption.[Pubmed:27559184]
Hosp Pharm. 2016 Jul;51(7):520-3.
The purpose of this feature is to heighten awareness of specific adverse drug reactions (ADRs), discuss methods of prevention, and promote reporting of ADRs to the US Food and Drug Administration's (FDA's) MedWatch program (800-FDA-1088). If you have reported an interesting, preventable ADR to MedWatch, please consider sharing the account with our readers. Write to Dr. Mancano at ISMP, 200 Lakeside Drive, Suite 200, Horsham, PA 19044 (phone: 215-707-4936; e-mail: mmancano@temple.edu). Your report will be published anonymously unless otherwise requested. This feature is provided by the Institute for Safe Medication Practices (ISMP) in cooperation with the FDA's MedWatch program and Temple University School of Pharmacy. ISMP is an FDA MedWatch partner.
Interactions of cephalexin with bovine serum albumin: displacement reaction and molecular docking.[Pubmed:27853676]
Bioimpacts. 2016;6(3):125-133.
Introduction: The drug-plasma protein interaction is a fundamental issue in guessing and checking the serious drug side effects related with other drugs. The purpose of this research was to study the interaction of Cephalexin with bovine serum albumin (BSA) and displacement reaction using site probes. Methods: The interaction mechanism concerning Cephalexin (CPL) with BSA was investigated using various spectroscopic methods and molecular modeling method. The binding sites number, n, apparent binding constant, K, and thermodynamic parameters, DeltaG(0), DeltaH(0), and DeltaS(0) were considered at different temperatures. To evaluate the experimental results, molecular docking modeling was calculated. Results: The distance, r=1.156 nm between BSA and CPL were found in accordance with the Forster theory of non-radiation energy transfer (FRET) indicating energy transfer occurs between BSA and CPL. According to the binding parameters and DeltaG(0)= negative values and DeltaS(0)= 28.275 j mol(-1)K(-1), a static quenching process is effective in the CPL-BSA interaction spontaneously. DeltaG(0) for the CPL-BSA complex obtained from the docking simulation is -28.99 kj mol(-1), which is close to experimental DeltaG of binding, -21.349 kj mol(-1) that indicates a good agreement between the results of docking methods and experimental data. Conclusion: The outcomes of spectroscopic methods revealed that the conformation of BSA changed during drug-BSA interaction. The results of FRET propose that CPL quenches the fluorescence of BSA by static quenching and FRET. The displacement study showed that phenylbutazon and ketoprofen displaced CPL, indicating that its binding site on albumin is site I and Gentamicin cannot be displaced from the binding site of CPL. All results of molecular docking method agreed with the results of experimental data.
Nasal carriage of methicillin-resistant Staphylococcus pseudintermedius in dogs treated with cephalexin monohydrate.[Pubmed:28042159]
Can Vet J. 2017 Jan;58(1):73-77.
This study aimed to investigate the nasal carriage of methicillin-resistant Staphylococcus pseudintermedius (MRSP) in dogs treated with oral Cephalexin monohydrate. Ten dogs with superficial pyoderma were monitored longitudinally for carriage of MRSP for up to 1 year after treatment; the strains were typed and antibiograms were determined. Methicillin-susceptible S. pseudintermedius (MSSP) was recovered prior to treatment in all dogs and could be isolated after 12 months in 1 dog. Methicillin-resistant Staphylococcus pseudintermedius was detected within 1 week of treatment in all dogs, and 3 clones represented by ST45, ST112, and ST181 were consistently present for up to 12 months after treatment. All MRSP isolates were resistant to at least 7 common antimicrobials. Oral Cephalexin monohydrate treatment selected for strains of multi-resistant MRSP, which were still present after 1 year.


