Delphinidin-3-O-rutinoside chlorideCAS# 15674-58-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15674-58-5 | SDF | Download SDF |
| PubChem ID | 192918 | Appearance | Dark, reddish powder |
| Formula | C27H31O16Cl | M.Wt | 647.0 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 3-O-Rutinosyldelphinidin chloride; Tulipanin chloride | ||
| Solubility | Soluble in methan | ||
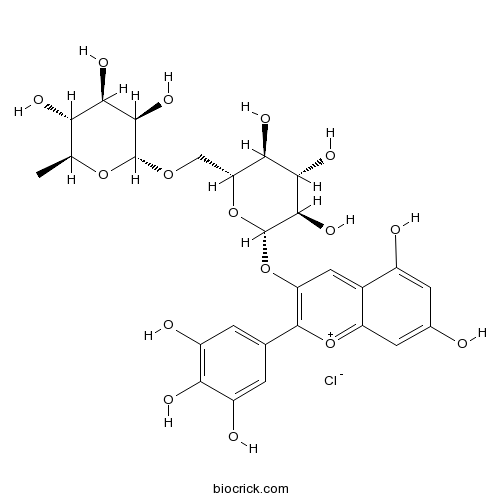
| Chemical Name | (2R,3R,4R,5R,6S)-2-[[(2R,3S,4S,5R,6S)-6-[5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chromenylium-3-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methoxy]-6-methyloxane-3,4,5-triol;chloride | ||
| SMILES | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=C([O+]=C4C=C(C=C(C4=C3)O)O)C5=CC(=C(C(=C5)O)O)O)O)O)O)O)O)O.[Cl-] | ||
| Standard InChIKey | ZOQQFMKYEOHRMC-KFOCXKDFSA-N | ||
| Standard InChI | InChI=1S/C27H30O16.ClH/c1-8-18(32)21(35)23(37)26(40-8)39-7-17-20(34)22(36)24(38)27(43-17)42-16-6-11-12(29)4-10(28)5-15(11)41-25(16)9-2-13(30)19(33)14(31)3-9;/h2-6,8,17-18,20-24,26-27,32,34-38H,7H2,1H3,(H4-,28,29,30,31,33);1H/t8-,17+,18-,20+,21+,22-,23+,24+,26+,27+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Delphinidin-3-O-rutinoside chloride has antioxidant activity. |

Delphinidin-3-O-rutinoside chloride Dilution Calculator

Delphinidin-3-O-rutinoside chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5456 mL | 7.728 mL | 15.456 mL | 30.9119 mL | 38.6399 mL |
| 5 mM | 0.3091 mL | 1.5456 mL | 3.0912 mL | 6.1824 mL | 7.728 mL |
| 10 mM | 0.1546 mL | 0.7728 mL | 1.5456 mL | 3.0912 mL | 3.864 mL |
| 50 mM | 0.0309 mL | 0.1546 mL | 0.3091 mL | 0.6182 mL | 0.7728 mL |
| 100 mM | 0.0155 mL | 0.0773 mL | 0.1546 mL | 0.3091 mL | 0.3864 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SNC 80
Catalog No.:BCC6785
CAS No.:156727-74-1
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- Org 20599
Catalog No.:BCC7470
CAS No.:156685-94-8
- 1,2,5-Trihydroxyxanthone
Catalog No.:BCN7585
CAS No.:156640-23-2
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Ryanodine
Catalog No.:BCC5742
CAS No.:15662-33-6
- 7,13-Dideacetyl-9,10-didebenzoyltaxchinin C
Catalog No.:BCN7670
CAS No.:156497-25-5
- 1-Hydroxytropacocaine
Catalog No.:BCN1919
CAS No.:156497-23-3
- Myricetin 3-O-galactoside
Catalog No.:BCN4703
CAS No.:15648-86-9
- α-Conotoxin ImI
Catalog No.:BCC5974
CAS No.:156467-85-5
- cis-Khellactone
Catalog No.:BCN3703
CAS No.:15645-11-1
- CWHM-12
Catalog No.:BCC5548
CAS No.:1564286-55-0
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
- Cephalexin
Catalog No.:BCC4646
CAS No.:15686-71-2
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
- N1,N11-Diethylnorspermine tetrahydrochloride
Catalog No.:BCC6654
CAS No.:156886-85-0
- Licofelone
Catalog No.:BCC4432
CAS No.:156897-06-2
- OSU 6162 hydrochloride
Catalog No.:BCC7424
CAS No.:156907-84-5
Genomic analysis of Isometamidium Chloride resistance in Trypanosoma congolense.[Pubmed:29032180]
Int J Parasitol Drugs Drug Resist. 2017 Dec;7(3):350-361.
Isometamidium Chloride (ISM) is one of the principal drugs used to counteract Trypanosoma congolense infection in livestock, both as a prophylactic as well as a curative treatment. However, numerous cases of ISM resistance have been reported in different African regions, representing a significant constraint in the battle against Animal African Trypanosomiasis. In order to identify genetic signatures associated with ISM resistance in T. congolense, the sensitive strain MSOROM7 was selected for induction of ISM resistance in a murine host. Administered ISM concentrations in immune-suppressed mice were gradually increased from 0.001 mg/kg to 1 mg/kg, the maximal dose used in livestock. As a result, three independent MSOROM7 lines acquired full resistance to this concentration after five months of induction, and retained this full resistant phenotype following a six months period without drug pressure. In contrast, parasites did not acquire ISM resistance in immune-competent animals, even after more than two years under ISM pressure, suggesting that the development of full ISM resistance is strongly enhanced when the host immune response is compromised. Genomic analyses comparing the ISM resistant lines with the parental sensitive line identified shifts in read depth at heterozygous loci in genes coding for different transporters and transmembrane products, and several of these shifts were also found within natural ISM resistant isolates. These findings suggested that the transport and accumulation of ISM inside the resistant parasites may be modified, which was confirmed by flow cytometry and ex vivo ISM uptake assays that showed a decrease in the accumulation of ISM in the resistant parasites.
Effects of Extended Storage of Chlorhexidine Gluconate and Benzalkonium Chloride Solutions on the Viability of Burkholderia cenocepacia.[Pubmed:29032643]
J Microbiol Biotechnol. 2017 Dec 28;27(12):2211-2220.
Chlorhexidine gluconate (CHX) and benzalkonium chloride (BZK) formulations are frequently used as antiseptics in healthcare and consumer products. Burkholderia cepacia complex (BCC) contamination of pharmaceutical products could be due to the use of contaminated water in the manufacturing process, over-diluted antiseptic solutions in the product, and the use of outdated products, which in turn reduces the antimicrobial activity of CHX and BZK. To establish a "safe use" period following opening containers of CHX and BZK, we measured the antimicrobial effects of CHX (2-10 mug/ml) and BZK (10-50 mug/ml) at sublethal concentrations on six strains of Burkholderia cenocepacia using chemical and microbiological assays. CHX (2, 4, and 10 mug/ml) and BZK (10, 20, and 50 mug/ml) stored for 42 days at 23 degrees C showed almost the same concentration and toxicity compared with freshly prepared CHX and BZK on B. cenocepacia strains. When 5 mug/ml CHX and 20 mug/ml BZK were spiked to six B. cenocepacia strains with different inoculum sizes (10(0) -10(5) CFU/ml), their toxic effects were not changed for 28 days. B. cenocepacia strains in diluted CHX and BZK were detectable at concentrations up to 10(2) CFU/ml after incubation for 28 days at 23 degrees C. Although abiotic and biotic changes in the toxicity of both antiseptics were not observed, our results indicate that B. cenocepacia strains could remain viable in CHX and BZK for 28 days, which in turn, indicates the importance of control measures to monitor BCC contamination in pharmaceutical products.
Chloride-Tolerant Gold(I)-Catalyzed Regioselective Hydrochlorination of Alkynes.[Pubmed:29034119]
ACS Catal. 2017 Oct 6;7(10):6798-6801.
We have developed a highly regioselective homogeneous gold(I)-catalyzed anti-hydrochlorination of unactivated alkynes at room temperature. We have overcome the incompatibility between conventional cationic gold catalysts and chloride by using a hydrogen-bonding activation of the Au-Cl bond. This approach is scalable, exhibits excellent functional group tolerance, and can be conducted in open air.
Heterogeneous electro-Fenton catalyst for 1-butylpyridinium chloride degradation.[Pubmed:29034428]
Environ Sci Pollut Res Int. 2019 Feb;26(4):3145-3156.
The application of the electro-Fenton process for organic compound mineralisation has been widely reported over the past years. However, operational problems related to the use of soluble iron salt as a homogeneous catalyst involve the development of novel catalysts that are able to operate in a wide pH range. For this purpose, polyvinyl alcohol-alginate beads, containing goethite as iron, were synthesised and evaluated as heterogeneous electro-Fenton catalyst for 1-butylpyridinium chloride mineralisation. The influence of catalyst dosage and pH solution on ionic liquid degradation was analysed, achieving almost total oxidation after 60 min under optimal conditions (2 g/L catalyst concentration and pH 3). The results showed good catalyst stability and reusability, although its effectiveness decreases slightly after three successive cycles. Furthermore, a plausible mineralisation pathway was proposed based on the oxidation byproducts determined by chromatographic techniques. Finally, the Microtox(R) test revealed notable detoxification after treatment which demonstrates high catalyst ability for pyridinium-based ionic liquid degradation by the electro-Fenton process.
Effect of choline chloride premedication on xylazine-induced hypoxaemia in sheep.[Pubmed:29033246]
Vet Anaesth Analg. 2017 Sep;44(5):1149-1155.
OBJECTIVE: To determine the anti-inflammatory efficacy of choline in vivo and in vitro and to investigate the anti-inflammatory mechanisms of choline. STUDY DESIGN: Randomized, controlled studies. ANIMALS: In vivo trials used 16 Romney sheep. In vitro experiments utilized RAW 264.7 mouse macrophage cells. METHODS: Hypoxaemia induced in 16 sheep by intravenous (IV) injection of 50 mug kg(-1) xylazine, an alpha-2 agonist, was measured in sheep at 0, 1 and 4 minutes using arterial blood gas analysis with and without 50 mg kg(-1) IV choline chloride premedication. Cell culture studies used enzyme-linked immunosorbent assay to measure the release of tumour necrosis factor (TNF-alpha) from lipopolysaccharide (LPS) stimulated macrophages with and without choline chloride premedication. TNF-alpha release was compared to thalidomide suppressed and untreated cells. RESULTS: Choline premedication in sheep mitigated a reduction in arterial partial pressure of oxygen (PaO2) but did not prevent development of clinically significant hypoxaemia. Decrease in mean PaO2 of choline treated sheep was 6.36 kPa (47.7 mmHg) compared to 9.81 kPa (73.6 mmHg) in control sheep. In vitro studies demonstrate that choline administered concurrent with LPS activation did not significantly suppress TNF-alpha expression but that treatment of cells with choline 10 minutes prior to LPS activation did significantly suppress TNF-alpha expression. Choline pretreated cells expressed 23.99 +/- 4.52 ng mg(-1) TNF-alpha while LPS only control cells expressed 33.83 +/- 3.20 ng mg(-1). CONCLUSIONS: Choline is able to prevent macrophage activation in vitro when administered prior to LPS activation and may reduce hypoxaemia in sheep developing pulmonary oedema after xylazine administration. This effect requires premedication with choline. CLINICAL RELEVANCE: Pharmacological manipulation of autonomic inflammatory responses holds promise for the treatment of inflammation. However, the complex cellular mechanisms involved in this reflex means that an adequate therapy should approach multiple pathways and mechanisms of the inflammatory response.


