Batimastat sodium saltAnticancer drug CAS# 130464-84-5 |
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- GM 6001
Catalog No.:BCC2119
CAS No.:142880-36-2
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130464-84-5 | SDF | Download SDF |
| PubChem ID | 59955182 | Appearance | Powder |
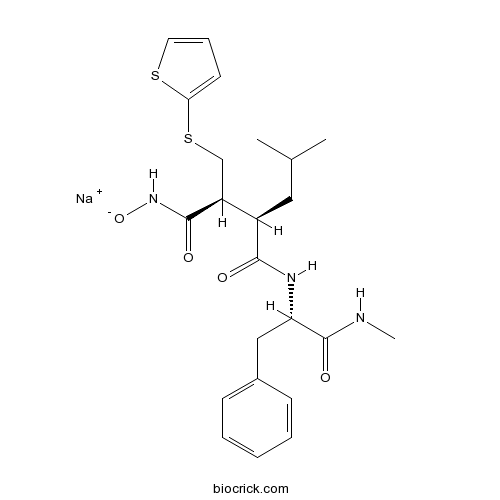
| Formula | C23H30N3NaO4S2 | M.Wt | 499.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BB-94 sodium salt | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | sodium;(2R,3S)-N-[(2S)-1-(methylamino)-1-oxo-3-phenylpropan-2-yl]-2-(2-methylpropyl)-N'-oxido-3-(thiophen-2-ylsulfanylmethyl)butanediamide | ||
| SMILES | CC(C)CC(C(CSC1=CC=CS1)C(=O)N[O-])C(=O)NC(CC2=CC=CC=C2)C(=O)NC.[Na+] | ||
| Standard InChIKey | VWABIWQKZWQAKG-WXLIBGKBSA-N | ||
| Standard InChI | InChI=1S/C23H30N3O4S2.Na/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16;/h4-11,15,17-19H,12-14H2,1-3H3,(H3-,24,25,26,27,28,29,30);/q-1;+1/t17-,18+,19+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Batimastat sodium salt is a potent broad spectrum MMP inhibitor with IC50 of 3, 4, 4, 6, and 20 nM for MMP-1, MMP-2, MMP-9, MMP-7 and MMP-3, respectively.In Vitro:Batimastat (BB-94) is a potent matrix metalloproteinase inhibitor, exhibits an unexpected mode of binding. Batimastat inhibits gelatinases A and B with IC50 values of 4 nM and 10 nM, respectively. The IC50 with the structurally similar collagenase Ht-d is 6 nM, which is comparable with values for MMP-1 (3 nM), MMP-8 (10 nM), and MMP-3 (20 nM)[2]. CD30 shedding from the cell line Karpas299 can effectively be blocked by the hydroxamic acidbased metalloproteinase inhibitor Batimastat (BB-94, IC50=230 nM)[3].In Vivo:Intraperitoneal administration of Batimastat (BB-94) effectively blocks growth of human ovarian carcinoma xenografts and murine melanoma metastasis and delays the growth of primary tumors in an orthotopic model of human breast cancer without cytotoxicity and without affecting mRNA levels[2]. Batimastat (BB-94) is a synthetic matrix metalloproteinase inhibitor that has shown antineoplastic and antiangiogenic activity in various tumor models. Treatment with Batimastat (60 mg/kg i.p. every other day, for a total of eight injections) concomitantly with Cisplatin (4 mg/kg i.v., every 7 days for a total of three injections) completely prevents growth and spread of both xenografts, and all animals are alive and healthy on day 200[4]. Kaplan-Meier analysis of survival (at 48 h) shows that animals treated with Batimastat (BB-94) have increased survival (95.2%) in comparison with controls (75%), and differences are almost statistically significant (p=0.064)[5]. Matrix density is analyzed in saline- or Batimastat (40 mg/kg)-pretreated animals 4 h after E2 administration, the time point at which collagen density is observed to be at its lowest after hormone treatment[6]. References: | |||||

Batimastat sodium salt Dilution Calculator

Batimastat sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0015 mL | 10.0076 mL | 20.0152 mL | 40.0304 mL | 50.038 mL |
| 5 mM | 0.4003 mL | 2.0015 mL | 4.003 mL | 8.0061 mL | 10.0076 mL |
| 10 mM | 0.2002 mL | 1.0008 mL | 2.0015 mL | 4.003 mL | 5.0038 mL |
| 50 mM | 0.04 mL | 0.2002 mL | 0.4003 mL | 0.8006 mL | 1.0008 mL |
| 100 mM | 0.02 mL | 0.1001 mL | 0.2002 mL | 0.4003 mL | 0.5004 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Batimastat (BB-94) is an anticancer drug that belongs to the family of drugs called angiogenesis inhibitors. Batimastat (BB-94) is a potent, broad spectrum matrix metalloprotease (MMP) inhibitor.
- Peucedanocoumarin III
Catalog No.:BCN3471
CAS No.:130464-57-2
- Peucedanocoumarin II
Catalog No.:BCN3435
CAS No.:130464-56-1
- Peucedanocoumarin I
Catalog No.:BCN3434
CAS No.:130464-55-0
- A-71623
Catalog No.:BCC7354
CAS No.:130408-77-4
- (-)-Catechin gallate(CG)
Catalog No.:BCN5330
CAS No.:130405-40-2
- Paulownin
Catalog No.:BCN6160
CAS No.:13040-46-5
- (RS)-MCPG disodium salt
Catalog No.:BCC7756
CAS No.:1303994-09-3
- CHPG Sodium salt
Catalog No.:BCC7755
CAS No.:1303993-73-8
- DL-AP5 Sodium salt
Catalog No.:BCC7753
CAS No.:1303993-72-7
- 2-Oxokolavenol
Catalog No.:BCN4716
CAS No.:130395-82-3
- Pungiolide A
Catalog No.:BCN8128
CAS No.:130395-54-9
- Decinnamoyltaxagifine
Catalog No.:BCN7329
CAS No.:130394-69-3
- A 68930 hydrochloride
Catalog No.:BCC7104
CAS No.:130465-39-3
- ent-11alpha-Hydroxyabieta-8(14),13(15)-dien-16,12alpha-olide
Catalog No.:BCN7330
CAS No.:130466-20-5
- L-655,708
Catalog No.:BCC7023
CAS No.:130477-52-0
- TC-F 2
Catalog No.:BCC6147
CAS No.:1304778-15-1
- SKF 96365 hydrochloride
Catalog No.:BCC6953
CAS No.:130495-35-1
- Cannabisin A
Catalog No.:BCC8138
CAS No.:130508-46-2
- Liriope muscari baily Saponins
Catalog No.:BCN2817
CAS No.:130551-41-6
- Mogroside III
Catalog No.:BCN3167
CAS No.:130567-83-8
- alpha-Terthienylmethanol
Catalog No.:BCN6161
CAS No.:13059-93-3
- Yangambin
Catalog No.:BCN6706
CAS No.:13060-14-5
- Nitidine chloride
Catalog No.:BCN4957
CAS No.:13063-04-2
- (R)-(+)-Corypalmine
Catalog No.:BCN2289
CAS No.:13063-54-2
Inhibitors of the p38 mitogen-activated kinase modulate IL-4 induction of low affinity IgE receptor (CD23) in human monocytes.[Pubmed:9834082]
J Immunol. 1998 Dec 1;161(11):6005-13.
CD23, the low affinity IgE receptor, is up-regulated on the surface of IL-4-treated B cells and monocytes and is immediately proteolytically processed, releasing soluble fragments of CD23. Here, we report that inhibitors of the p38 mitogen-activated kinase (p38 MAPK), SK&F 86002 or the more selective inhibitor, SB 203580, reduce the levels of soluble CD23 formed by IL-4-stimulated human monocytes or the human monocytic cell line, U937. In contrast to compounds such as the metalloprotease inhibitor batimastat ([4-(N-hydroxyamino)-2-(R)-isobutyl-3-(S)-(2-thiophenethiomethyl)s uccinyl]-(S)-phenylalanine-N-methylamide, sodium salt), p38 MAPK inhibitors do not directly inhibit proteolytic processing of CD23. Further, evaluation of surface intact CD23 (iCD23) by flow cytometry demonstrated that SK&F 86002 and SB 203580 reduced the surface expression of iCD23 in a concentration-dependent fashion, while batimastat increased the surface expression of iCD23. The decrease in surface iCD23 was accompanied by a decrease in total cell-associated CD23 protein levels but not CD23 mRNA. IL-4 induced a late (>4-h) increase in p38 MAPK activity and corresponding activation of its substrate MAPKAPK-2. This activation was blocked by addition of SB 203580 before IL-4 induction, in parallel with the inhibition of CD23 expression. Modulation of CD23 by antibodies has been shown to alleviate the symptoms of murine collagen-induced arthritis, implicating CD23 as an important proinflammatory agent. These data show that in addition to the known cytokine inhibitory actions of SK&F 86002 and SB 203580, they also confer an additional potential anti-inflammatory activity through modulation of CD23 expression.


