A-71623CCKA receptor agonist CAS# 130408-77-4 |
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- 1-Azakenpaullone
Catalog No.:BCC5332
CAS No.:676596-65-9
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- AT7867
Catalog No.:BCC2536
CAS No.:857531-00-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130408-77-4 | SDF | Download SDF |
| PubChem ID | 121964 | Appearance | Powder |
| Formula | C44H56N8O9 | M.Wt | 840.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 20mM PBS buffer | ||
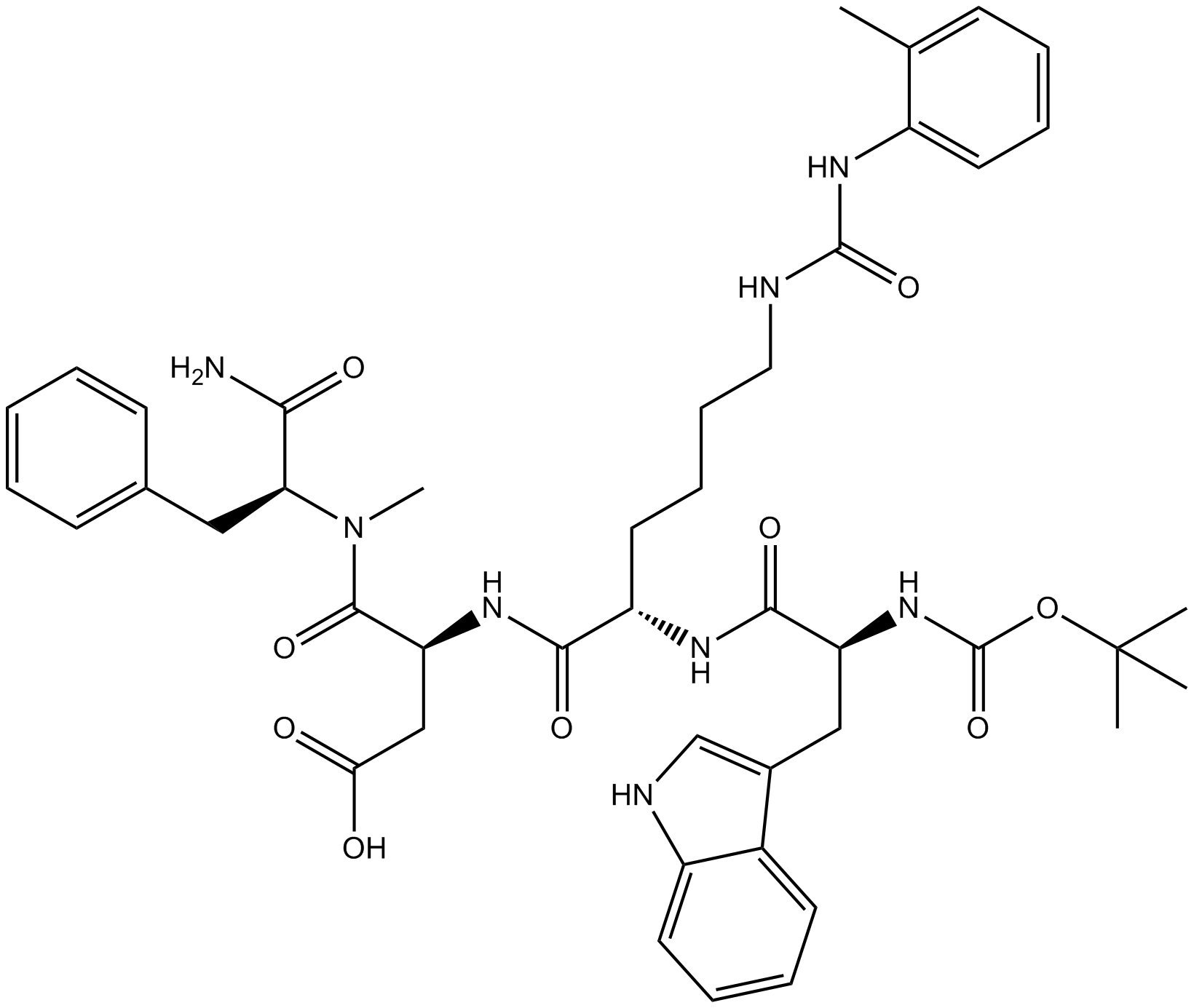
| Sequence | XWKDF (Modifications: Trp-1 = Boc-Trp, Lys-3 = Lys(Tac), Phe-5 = N-methyl-Phe & C-terminal amide) | ||
| Chemical Name | (3S)-4-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]-methylamino]-3-[[(2S)-2-[[(2S)-3-(1H-indol-3-yl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoyl]amino]-6-[(2-methylphenyl)carbamoylamino]hexanoyl]amino]-4-oxobutanoic acid | ||
| SMILES | CC1=CC=CC=C1NC(=O)NCCCCC(C(=O)NC(CC(=O)O)C(=O)N(C)C(CC2=CC=CC=C2)C(=O)N)NC(=O)C(CC3=CNC4=CC=CC=C43)NC(=O)OC(C)(C)C | ||
| Standard InChIKey | KNHCBYMGWWTGSO-ZYADHFCISA-N | ||
| Standard InChI | InChI=1S/C44H56N8O9/c1-27-15-9-11-19-31(27)50-42(59)46-22-14-13-21-33(39(56)49-35(25-37(53)54)41(58)52(5)36(38(45)55)23-28-16-7-6-8-17-28)48-40(57)34(51-43(60)61-44(2,3)4)24-29-26-47-32-20-12-10-18-30(29)32/h6-12,15-20,26,33-36,47H,13-14,21-25H2,1-5H3,(H2,45,55)(H,48,57)(H,49,56)(H,51,60)(H,53,54)(H2,46,50,59)/t33-,34-,35-,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent CCK1 agonist (IC50 = 3.7 nM) with 1200-fold selectivity over the CCK2 receptor. Suppresses food intake following central or peripheral administration. |

A-71623 Dilution Calculator

A-71623 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A-71623 is a selective agonist of CCKA receptor with an IC50 value of 3.7 nM in guinea pig pancreas [1, 2].
CCKA receptors belong to a subtype of cholecystokinin (CCK) receptors in the brain. CCK is a type of neuropeptide present throughout the central nervous system. CCK can act as a neurotransmitter in both normal and abnormal brain. CCK receptors exist in two forms in the brain. Another subtype of CCK receptors is CCKB subtype [1].
In NCI-H345 cells possessing CCKB/gastrin receptors, A-71623 was weak and behaved as a partial agonist in calcium studies [2]. A-71623 had very low affinity to CCK binding sites in C6 cells with an IC50 value of 1236 ± 81 nM [3]. It is hard to find the CCKA response result of the application of A-71623 in cells.
In radioligand binding assays, A-71623 showed IC50 values of 3.7 nM for CCKA in guinea pig pancreas and 4500 nM for CCKB in cerebral cortex. Data showed that A-71623 was an agonist in stimulating the release of pancreatic amylase, and this stimulatory effect was potently inhibited by L-364,718, a CCKA antagonist. Data showed that A-71623 acted as a full agonist in stimulating the breakdown of phosphoinositide in pancreas. In the ileum, A-71623 was also a potent agonist in stimulating CCKA receptors. In guinea pig gastric glands, the affinity of A-71623 for the CCK-B/gastrin receptor was 11 µM. This result demonstrated that A-71623 should be a potent and selective agonist at CCKA receptors [2].
References:
[1]. Gracey DJ, Bell R, King DJ. Differential effects of the CCKA receptor ligands PD-140,548 and A-71623 on latent inhibition in the rat. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2002, 26(3): 497-504.
[2]. Lin CW, Shiosaki K, Miller TR, et al. Characterization of two novel cholecystokinin tetrapeptide (30-33) analogues, A-71623 and A-70874, that exhibit high potency and selectivity for cholecystokinin-A receptors. Molecular pharmacology, 1991, 39(3): 346-351.
[3]. Kaufmann R, Lindschau C, Scho T, et al. Type B cholecystokinin receptors on rat glioma C6 cells. Binding studies and measurement of intracellular calcium mobilization. Brain research, 1994, 639(1): 109-114.
- (-)-Catechin gallate(CG)
Catalog No.:BCN5330
CAS No.:130405-40-2
- Paulownin
Catalog No.:BCN6160
CAS No.:13040-46-5
- (RS)-MCPG disodium salt
Catalog No.:BCC7756
CAS No.:1303994-09-3
- CHPG Sodium salt
Catalog No.:BCC7755
CAS No.:1303993-73-8
- DL-AP5 Sodium salt
Catalog No.:BCC7753
CAS No.:1303993-72-7
- 2-Oxokolavenol
Catalog No.:BCN4716
CAS No.:130395-82-3
- Pungiolide A
Catalog No.:BCN8128
CAS No.:130395-54-9
- Decinnamoyltaxagifine
Catalog No.:BCN7329
CAS No.:130394-69-3
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- AC 45594
Catalog No.:BCC7544
CAS No.:13037-86-0
- MI-773 (SAR405838)
Catalog No.:BCC5648
CAS No.:1303607-60-4
- MI-773
Catalog No.:BCC5155
CAS No.:1303607-07-9
- Peucedanocoumarin I
Catalog No.:BCN3434
CAS No.:130464-55-0
- Peucedanocoumarin II
Catalog No.:BCN3435
CAS No.:130464-56-1
- Peucedanocoumarin III
Catalog No.:BCN3471
CAS No.:130464-57-2
- Batimastat sodium salt
Catalog No.:BCC2075
CAS No.:130464-84-5
- A 68930 hydrochloride
Catalog No.:BCC7104
CAS No.:130465-39-3
- ent-11alpha-Hydroxyabieta-8(14),13(15)-dien-16,12alpha-olide
Catalog No.:BCN7330
CAS No.:130466-20-5
- L-655,708
Catalog No.:BCC7023
CAS No.:130477-52-0
- TC-F 2
Catalog No.:BCC6147
CAS No.:1304778-15-1
- SKF 96365 hydrochloride
Catalog No.:BCC6953
CAS No.:130495-35-1
- Cannabisin A
Catalog No.:BCC8138
CAS No.:130508-46-2
- Liriope muscari baily Saponins
Catalog No.:BCN2817
CAS No.:130551-41-6
- Mogroside III
Catalog No.:BCN3167
CAS No.:130567-83-8
CCK-A receptor selective antagonists derived from the CCK-A receptor selective tetrapeptide agonist Boc-Trp-Lys(Tac)-Asp-MePhe-NH2 (A-71623).[Pubmed:7837233]
J Med Chem. 1995 Jan 6;38(1):207-11.
Analogs of the CCK-A receptor selective agonist Boc-Trp-Lys(Tac)-Asp-MePhe-NH2 (A-71623) were prepared in which the lysine residue was replaced with L-4-aminophenylalanine and D-or L-3-aminophenylalanine. These new analogs were moderately potent antagonists of CCK-8 in the isolated guinea pig gallbladder with exceptional CCK-A receptor selectivity as evaluated in membrane preparations from CHO K1 cells stably transfected with human CCK-A and CCK-B receptors.
Alternate drug delivery routes for A-71623, a potent cholecystokinin-A receptor agonist tetrapeptide.[Pubmed:8894966]
J Drug Target. 1996;4(2):69-78.
A-71623 (BOC-Trp-Lys(epsilon-N-2-methylphenylaminocarbonyl)- Asp-(N-methyl)-Phe-NH2) is a tetrapeptide which has high affinity and selectivity for cholecystokinin receptors; it is a potent appetite suppresser in animal studies. Because of its low (< 1%) oral bioavailability, studies were performed to assess the feasibility of delivery of A-71623 by pulmonary, sublingual, and transdermal routes of administration. The pKa was determined to be 4.2 by spectrophotometric titration; aqueous solubility is increased by increasing pH and by increasing ethanol content. The solubility of A-71623 in ethanol/propellant mixtures was investigated; solubility ranged from 1.0 to 2.5 mg/mL in mixtures of ethanol, propellant 11 (trichlorofluoromethane), and propellant 12 (dichlorodifluoromethane). The log apparent octanol/water partition coefficient was 2.8 at pH 5 and 1.0 at pH 8. Maximum stability at 70 degrees C was seen in the range of pH values of 5.5-7.5; hydrolysis of the N-terminal BOC group appears to be the primary route of degradation. Increasing ethanol content increases the stability; Arrhenius analysis indicated a t90 of 150 days under ambient conditions in 25% ethanol. Intratracheal delivery of 3 mumol/kg A-71623 in 50% ethanol to rats showed rapid and efficient absorption of drug from the lungs, with a Cmax of 2.7 microM and an AUC of 85 microM*min. Similar studies in dogs showed bioavailabilities of 59% and 46% for 2 and 3 mumol/kg intratracheal doses, respectively, relative to intravenous administration. Sublingual administration of 1 mumol/kg A-71623 in a vehicle of 80% ethanol/2% Klucel/2.5% peppermint oil gave high prolonged plasma levels of A-71623, with a Cmax of 0.37 microM, indicating high bioavailability and favorable partitioning and distribution effects from the sublingual cavity for this formulation. Transdermal delivery was examined by in vitro diffusion through human skin; the permeability coefficient of A-71623 in 40% ethanol was 2.6 x 10(-5) cm/hr, suggesting that transdermal delivery of up to 2 mg/day may be feasible. In conclusion, the results provide preliminary indications that delivery of efficacious doses of A-71623, and perhaps other CCK analogs, by alternate routes of delivery is probably feasible.
A-71623, a selective CCK-A receptor agonist, suppresses food intake in the mouse, dog, and monkey.[Pubmed:1513850]
Pharmacol Biochem Behav. 1992 Aug;42(4):699-704.
The anorectic actions of cholecystokinin (CCK)-8 and of a selective CCK-A agonist, A-71623, were examined in CD1 mice, beagle dogs, and cynomolgus monkeys. A-71623 suppressed intakes in all species tested, and the effects were blocked by a selective CCK-A antagonist, A-70104. In the dog only, CCK-8 was more potent on a molar basis compared to A-71623, although the effects of both CCK agonists were more short-lived in the dog compared to the other species tested. Our results support other evidence suggesting that the anorectic actions of exogenous application of CCK-8 in these species are mediated via stimulation of the CCK-A receptor subtype.
Differential effects of the CCKA receptor ligands PD-140,548 and A-71623 on latent inhibition in the rat.[Pubmed:11999900]
Prog Neuropsychopharmacol Biol Psychiatry. 2002 Apr;26(3):497-504.
Latent inhibition (LI) is a behavioural paradigm in which repeated exposure to a stimulus without consequence inhibits the formation of any new associations with that stimulus. To the extent that LI reflects a process of leaming to ignore irrelevant stimuli, disrupted LI has been suggested as an animal model for the attentional deficits observed in schizophrenia. The antipsychotic potential of cholecystokinin (CCK) stems from its colocalization with dopamine (DA) in the mesolimbic pathway, where it demonstrates both excitatory and inhibitory effects on dopaminergic activity. This may be explained by mediation through different receptor subtypes. A variety of hypotheses has emerged regarding the potential clinical application of subtype-selective CCK-based drugs. The present experiments examined the effects on LI of two selective CCK(A) ligands: PD-140,548 (a CCK(A) antagonist, Experiment 1: 0.001, 0.01, and 0.1 mg/kg) and A-71623 (a CCK(A) agonist, Experiment 2: 0.02, 0.05, and 0.1 mg/kg). In both experiments, the effects of haloperidol (0.1 mg/kg) were also investigated. Animals receiving 0.1 mg/kg of haloperidol or 0.001 or 0.1 mg/kg (but not 0.01 mg/kg) of PD-140,548 treated the preexposed stimulus as irrelevant after a low number of preexposures. In contrast, no facilitatory effect on LI was detectable at any of the A-71623 doses. The finding that A-71623 failed to enhance LI indicates that it is unlikely that this compound would have any antipsychotic effect within the clinical setting. Considering the facilitatory effect exerted by PD-140,548 on LI, it is probable that the inhibition of CCK activity might prove a more promising strategy for the pharmacological treatment of schizophrenia.


