NemorubicinDoxorubicin analog,anticancer drug CAS# 108852-90-0 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- 7-Xylosyltaxol
Catalog No.:BCN5341
CAS No.:90332-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108852-90-0 | SDF | Download SDF |
| PubChem ID | 65907 | Appearance | Powder |
| Formula | C32H37NO13 | M.Wt | 643.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Methoxymorpholinyldoxorubicin; PNU 152243; PNU-152243A | ||
| Solubility | DMSO : ≥ 47 mg/mL (73.02 mM) *"≥" means soluble, but saturation unknown. | ||
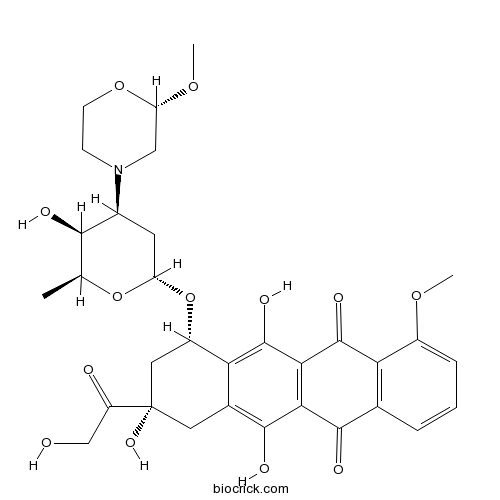
| Chemical Name | (7S,9S)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7-[(2R,4S,5S,6S)-5-hydroxy-4-[(2S)-2-methoxymorpholin-4-yl]-6-methyloxan-2-yl]oxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | ||
| SMILES | CC1C(C(CC(O1)OC2CC(CC3=C(C4=C(C(=C23)O)C(=O)C5=C(C4=O)C=CC=C5OC)O)(C(=O)CO)O)N6CCOC(C6)OC)O | ||
| Standard InChIKey | CTMCWCONSULRHO-UHQPFXKFSA-N | ||
| Standard InChI | InChI=1S/C32H37NO13/c1-14-27(36)17(33-7-8-44-22(12-33)43-3)9-21(45-14)46-19-11-32(41,20(35)13-34)10-16-24(19)31(40)26-25(29(16)38)28(37)15-5-4-6-18(42-2)23(15)30(26)39/h4-6,14,17,19,21-22,27,34,36,38,40-41H,7-13H2,1-3H3/t14-,17-,19-,21-,22-,27+,32-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nemorubicin is an anticancer drug with IC50 value of 0.08 μM. | |||||
| Targets | cancer | |||||
| IC50 | 0.08 μM | |||||

Nemorubicin Dilution Calculator

Nemorubicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5537 mL | 7.7683 mL | 15.5366 mL | 31.0733 mL | 38.8416 mL |
| 5 mM | 0.3107 mL | 1.5537 mL | 3.1073 mL | 6.2147 mL | 7.7683 mL |
| 10 mM | 0.1554 mL | 0.7768 mL | 1.5537 mL | 3.1073 mL | 3.8842 mL |
| 50 mM | 0.0311 mL | 0.1554 mL | 0.3107 mL | 0.6215 mL | 0.7768 mL |
| 100 mM | 0.0155 mL | 0.0777 mL | 0.1554 mL | 0.3107 mL | 0.3884 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nemorubicin is an anticancer drug with IC50 value of 0.08μM [1].
Nemorubicin is a morpholinyl analog of doxorubicin and is developed to be an anticancer drug. It is less cardiotoxic and more cytotoxic against multidrug-resistant tumor cells. The SRB in vitro assay shows nemorubicin can inhibit the proliferation of HCC cell lines with average IC50 value of 0.08μM. It is much more potent(from 10 to 380 times) than other anticancer drugs. The treatments of nemorubicin at doses of both 25μg/kg and 50μg/kg cause growth inhibition of human HCC xenografts BEL-7402 and Zip-177 in nude mice. When the dose is up to 100μg/kg, the high toxicity causes the death of mice. In phase I study, nemorubicin shows a terminal half-life in eight patients of 11.1±10.8 h. And the hematotoxicity tests show a <70% colony growth inhibition without correlation between the growth inhibition effect and the degree of myelotoxicity in the same patient. Besides that, nausea and vomiting is a limit of the application of nemorubicin [1, 2].
References:
[1] Shengtao Yuan, Xiongwen Zhang, Lijuan Lu, Chenghui Xu, Weiyi Yang and Jian Ding. Anticancer activity of methoxymorpholinyl doxorubicin (PNU 152243) on human hepatocellular carcinoma. Anti-Cancer Drugs. 2004, 15(6): 641-646.
[2] Cristiana Sessa, Massimo Zucchetti Michele Ghielmini, Jean Bauer, Maurizio D'Incalci Jolanda de Jong, Huguette Naegele, Simona Rossi Maria Adele Pacciarini, Letizia Domenigoni Franco Cavalli. Cancer Chemother Pharmacol. 1999, 44:403-410.
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- Mizolastine
Catalog No.:BCC4521
CAS No.:108612-45-9
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- Lumichrome
Catalog No.:BCN7083
CAS No.:1086-80-2
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
The interaction of nemorubicin metabolite PNU-159682 with DNA fragments d(CGTACG)(2), d(CGATCG)(2) and d(CGCGCG)(2) shows a strong but reversible binding to G:C base pairs.[Pubmed:23154079]
Bioorg Med Chem. 2012 Dec 15;20(24):6979-88.
The antitumor anthracycline Nemorubicin is converted by human liver microsomes to a major metabolite, PNU-159682 (PNU), which was found to be much more potent than its parent drug toward cultured tumor cells and in vivo tumor models. The mechanism of action of Nemorubicin appears different from other anthracyclines and until now is the object of studies. In fact PNU is deemed to play a dominant, but still unclear, role in the in vivo antitumor activity of Nemorubicin. The interaction of PNU with the oligonucleotides d(CGTACG)(2), d(CGATCG)(2) and d(CGCGCG)(2) was studied with a combined use of (1)H and (31)P NMR spectroscopy and by ESI-mass experiments. The NMR studies allowed to establish that the intercalation between the base pairs of the duplex leads to very stable complexes and at the same time to exclude the formation of covalent bonds. Melting experiments monitored by NMR, allowed to observe with high accuracy the behaviour of the imine protons with temperature, and the results showed that the re-annealing occurs after melting. The formation of reversible complexes was confirmed by HPLC-tandem mass spectra, also combined with endonuclease P1digestion. The MS/MS spectra showed the loss of neutral PNU before breaking the double helix, a behaviour typical of intercalators. After digestion with the enzyme, the spectra did not show any compound with PNU bound to the bases. The evidence of a reversible process appears from both proton and phosphorus NOESY spectra of PNU bound to d(CGTACG)(2) and to d(CGATCG)(2). The dissociation rate constants (k(off)) of the slow step of the intercalation process, measured by (31)P NMR NOE-exchange experiments, showed that the kinetics of the process is slower for PNU than for doxorubicin and Nemorubicin, leading to a 10- to 20-fold increase of the residence time of PNU into the intercalation sites, with respect to doxorubicin. A relevant number of NOE interactions allowed to derive a model of the complexes in solution from restrained MD calculations. The conformation of PNU bound to the oligonucleotides was also derived from the coupling constant values.
Virtual Cross-Linking of the Active Nemorubicin Metabolite PNU-159682 to Double-Stranded DNA.[Pubmed:28068470]
Chem Res Toxicol. 2017 Feb 20;30(2):614-624.
The DNA alkylating mechanism of PNU-159682 (PNU), a highly potent metabolite of the anthracycline Nemorubicin, was investigated by gel-electrophoretic, HPLC-UV, and micro-HPLC/mass spectrometry (MS) measurements. PNU quickly reacted with double-stranded oligonucleotides, but not with single-stranded sequences, to form covalent adducts which were detectable by denaturing polyacrylamide gel electrophoresis (DPAGE). Ion-pair reverse-phase HPLC-UV analysis on CG rich duplex sequences having a 5'-CCCGGG-3' central core showed the formation of two types of adducts with PNU, which were stable and could be characterized by micro-HPLC/MS. The first type contained one alkylated species (and possibly one reversibly bound species), and the second contained two alkylated species per duplex DNA. The covalent adducts were found to produce effective bridging of DNA complementary strands through the formation of virtual cross-links reminiscent of those produced by classical anthracyclines in the presence of formaldehyde. Furthermore, the absence of reactivity of PNU with CG-rich sequence containing a TA core (CGTACG), and the minor reactivity between PNU and CGC sequences (TACGCG.CGCGTA) pointed out the importance of guanine sequence context in modulating DNA alkylation.
Nemorubicin and doxorubicin bind the G-quadruplex sequences of the human telomeres and of the c-MYC promoter element Pu22.[Pubmed:26922833]
Biochim Biophys Acta. 2016 Jun;1860(6):1129-38.
BACKGROUND: Intra-molecular G-quadruplex structures are present in the guanine rich regions of human telomeres and were found to be prevalent in gene promoters. More recently, the targeting of c-MYC transcriptional control has been suggested, because the over expression of the c-MYC oncogene is one of the most common aberration found in a wide range of human tumors. METHODS: The interaction of Nemorubicin and doxorubicin with DNA G-quadruplex structures has been studied by NMR, ESI-MS and molecular modelling, in order to obtain further information about the complex and the multiple mechanisms of action of these drugs. RESULTS AND CONCLUSIONS: Nemorubicin intercalates between A3 and G4 of d(TTAGGGT)4 and form cap-complex at the G6pT7 site. The presence of the adenine in this sequence is important for the stabilization of the complex, as was shown by the interaction with d(TTGGGTT)4 and d(TTTGGGT)4, which form only a 1:1 complex. The interaction of doxorubicin with d(TTAGGGT)4 is similar, but the complex appears less stable. Nemorubicin also binds with high efficiency the c-MYC G-quadruplex sequence Pu22, to form a very well defined complex. Two Nemorubicin molecules bind to the 3'-end and to the 5'-end, forming an additional plane of stacking over each external G-tetrad. The wild type c-MYCPu22 sequence forms with Nemorubicin the same complex. GENERAL SIGNIFICANCE: Nemorubicin and doxorubicin, not only intercalate into the duplex DNA, but also result in significant ligands for G-quadruplex DNA segments, stabilizing their structure; this may in part explain the multiple mechanisms of action of their antitumor activity.
In vitro hepatic conversion of the anticancer agent nemorubicin to its active metabolite PNU-159682 in mice, rats and dogs: a comparison with human liver microsomes.[Pubmed:18671948]
Biochem Pharmacol. 2008 Sep 15;76(6):784-95.
We recently demonstrated that Nemorubicin (MMDX), an investigational antitumor drug, is converted to an active metabolite, PNU-159682, by human liver cytochrome P450 (CYP) 3A4. The objectives of this study were: (1) to investigate MMDX metabolism by liver microsomes from laboratory animals (mice, rats, and dogs of both sexes) to ascertain whether PNU-159682 is also produced in these species, and to identify the CYP form(s) responsible for its formation; (2) to compare the animal metabolism of MMDX with that by human liver microsomes (HLMs), in order to determine which animal species is closest to human beings; (3) to explore whether differences in PNU-159682 formation are responsible for previously reported species- and sex-related differences in MMDX host toxicity. The animal metabolism of MMDX proved to be qualitatively similar to that observed with HLMs since, in all tested species, MMDX was mainly converted to PNU-159682 by a single CYP3A form. However, there were marked quantitative inter- and intra-species differences in kinetic parameters. The mouse and the male rat exhibited V(max) and intrinsic metabolic clearance (CL(int)) values closest to those of human beings, suggesting that these species are the most suitable animal models to investigate MMDX biotransformation. A close inverse correlation was found between MMDX CL(int) and previously reported values of MMDX LD(50) for animals of the species, sex and strain tested here, indicating that differences in the in vivo toxicity of MMDX are most probably due to sex- and species-related differences in the extent of PNU-159682 formation.


