GSK-923295CENP-E inhibitor,small-molecule CAS# 1088965-37-0 |
- MPI-0479605
Catalog No.:BCC5347
CAS No.:1246529-32-7
- Kif15-IN-1
Catalog No.:BCC5152
CAS No.:672926-32-8
- Kif15-IN-2
Catalog No.:BCC5153
CAS No.:672926-33-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1088965-37-0 | SDF | Download SDF |
| PubChem ID | 46898058 | Appearance | Powder |
| Formula | C32H38ClN5O4 | M.Wt | 592.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 30 mg/mL (50.66 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
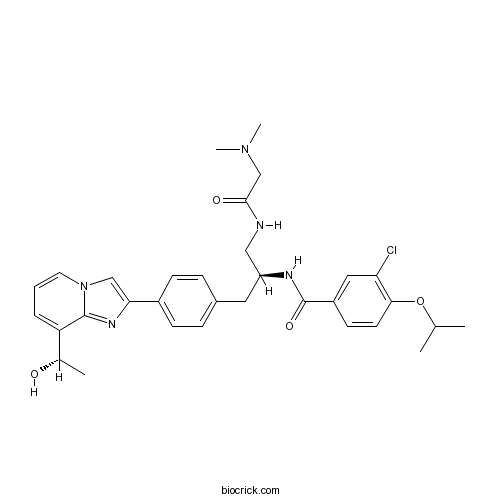
| Chemical Name | 3-chloro-N-[(2S)-1-[[2-(dimethylamino)acetyl]amino]-3-[4-[8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl]phenyl]propan-2-yl]-4-propan-2-yloxybenzamide | ||
| SMILES | CC(C)OC1=C(C=C(C=C1)C(=O)NC(CC2=CC=C(C=C2)C3=CN4C=CC=C(C4=N3)C(C)O)CNC(=O)CN(C)C)Cl | ||
| Standard InChIKey | WHMXDBPHBVLYRC-OFVILXPXSA-N | ||
| Standard InChI | InChI=1S/C32H38ClN5O4/c1-20(2)42-29-13-12-24(16-27(29)33)32(41)35-25(17-34-30(40)19-37(4)5)15-22-8-10-23(11-9-22)28-18-38-14-6-7-26(21(3)39)31(38)36-28/h6-14,16,18,20-21,25,39H,15,17,19H2,1-5H3,(H,34,40)(H,35,41)/t21-,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GSK-923295 is a small-molecule inhibitor of the mitotic kinesin centromere-associated protein E (CENP-E) with Ki value of 3.2 nM. | |||||
| Targets | CENP-E | |||||
| IC50 | 3.2 nM (Ki) | |||||

GSK-923295 Dilution Calculator

GSK-923295 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6888 mL | 8.4439 mL | 16.8879 mL | 33.7758 mL | 42.2197 mL |
| 5 mM | 0.3378 mL | 1.6888 mL | 3.3776 mL | 6.7552 mL | 8.4439 mL |
| 10 mM | 0.1689 mL | 0.8444 mL | 1.6888 mL | 3.3776 mL | 4.222 mL |
| 50 mM | 0.0338 mL | 0.1689 mL | 0.3378 mL | 0.6755 mL | 0.8444 mL |
| 100 mM | 0.0169 mL | 0.0844 mL | 0.1689 mL | 0.3378 mL | 0.4222 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK923295 is a specific inhibitor of CENP-E kinesin motor with IC50 value of 3.2 nM [1].
Centromere-associated protein E (CENP-E) is a mitotic kinesin, which is the connect of mitosis process with the mitotic checkpoint signaling. It Interacts with spindle microtubules and contribute to the chromosome alignment, and thus it regulates the cell-cycle transition from metaphase to anaphase.
When asynchronous cultured cells were exposed to GSK923295, the penetrant cell-cycle delay in mitosis was observed, which accompanied with morphological changes similar to RNAi-mediated knockdown of CENP-E mRNA. It indicated a significant inhibition of CENP-E by GSK923295. In the presence of GSK9232195, CENP-E microtubule (MT)-stimulated ATPase showed a dramatic slowing of release of ADP and Pi, where the ATP-bound form was stabilized and the activity of CENP-E was inhibited. This observation suggested GSK923295 inhibited CENP-E via the suppression of MT-stimulated ATPase [1].
In mouse model, mice bearing xenografts of the Colo205 colon tumor cell line were administered with GSK923295 of 125 mg/kg. The result showed GSK923295-induced cell-cycle changes of tumor cells and increased scattered apoptosis body. Additionally, long-term study via measuring tumor volume revealed significant antitumor activity of GSK923295, in a manner of dose-dependent [1].
Reference:
[1] Wood K W et al. , Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. 2010, 107 (13): 5839-5844.
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
Small Molecules Identified from a Quantitative Drug Combinational Screen Resensitize Cisplatin's Response in Drug-Resistant Ovarian Cancer Cells.[Pubmed:29982103]
Transl Oncol. 2018 Aug;11(4):1053-1064.
Drug resistance to chemotherapy occurs in many ovarian cancer patients resulting in failure of treatment. Exploration of drug resistance mechanisms and identification of new therapeutics that overcome the drug resistance can improve patient prognosis. Following a quantitative combination screen of 6060 approved drugs and bioactive compounds in a cisplatin-resistant A2780-cis ovarian cancer cell line, 38 active compounds with IC50s under 1 muM suppressed the growth of cisplatin-resistant ovarian cancer cells. Among these confirmed compounds, CUDC-101, OSU-03012, oligomycin A, VE-821, or Torin2 in a combination with cisplatin restored cisplatin's apoptotic response in the A2780-cis cells, while SR-3306, GSK-923295, SNX-5422, AT-13387, and PF-05212384 directly suppressed the growth of A2780-cis cells. One of the mechanisms for overcoming cisplatin resistance in these cells is mediated by the inhibition of epidermal growth factor receptor (EGFR), though not all the EGFR inhibitors are equally active. The increased levels of total EGFR and phosphorylated-EGFR (p-EGFR) in the A2780-cis cells were reduced after the combined treatment of cisplatin with EGFR inhibitors. In addition, a knockdown of EGFR mRNA reduced cisplatin resistance in the A2780-cis cells. Therefore, the top active compounds identified in this work can be studied further as potential treatments for cisplatin-resistant ovarian cancer. The quantitative combinational screening approach is a useful method for identifying effective compounds and drug combinations against drug-resistant cancer cells.


