MK 6096CAS# 1088991-73-4 |
- Zafirlukast
Catalog No.:BCC4881
CAS No.:107753-78-6
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- LY 255283
Catalog No.:BCC7290
CAS No.:117690-79-6
- Cinalukast
Catalog No.:BCC7244
CAS No.:128312-51-6
- Leukotriene B4
Catalog No.:BCC7322
CAS No.:71160-24-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1088991-73-4 | SDF | Download SDF |
| PubChem ID | 25128145 | Appearance | Powder |
| Formula | C24H25FN4O2 | M.Wt | 420.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Filorexant | ||
| Solubility | DMSO : 12.61 mg/mL (29.99 mM; Need ultrasonic) | ||
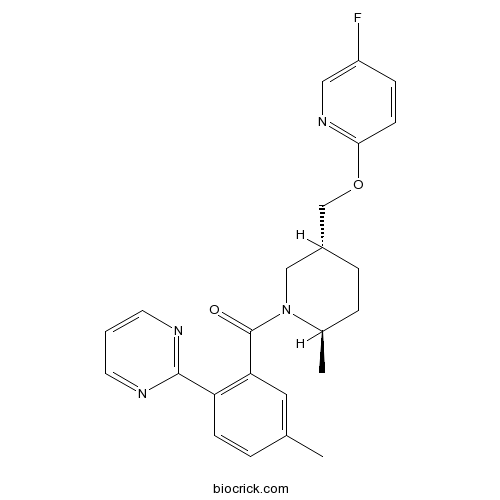
| Chemical Name | [(2R,5R)-5-[(5-fluoropyridin-2-yl)oxymethyl]-2-methylpiperidin-1-yl]-(5-methyl-2-pyrimidin-2-ylphenyl)methanone | ||
| SMILES | CC1CCC(CN1C(=O)C2=C(C=CC(=C2)C)C3=NC=CC=N3)COC4=NC=C(C=C4)F | ||
| Standard InChIKey | NPFDWHQSDBWQLH-QZTJIDSGSA-N | ||
| Standard InChI | InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK-6096(Filorexant) is an orally bioavailable potent and selective reversible antagonist of OX1 and OX2 receptor(<3 nM in binding).
IC50 value: < 3 nM(binding Ki) [1]
Target: Orexin receptor antagonist
in vitro: In radioligand binding and functional cell based assays MK-6096 demonstrated potent binding and antagonism of both human OX(1)R and OX(2)R (<3 nM in binding, 11 nM in FLIPR), with no significant off-target activities against a panel of >170 receptors and enzymes. MK-6096 occupies 90% of human OX(2)Rs expressed in transgenic rats at a plasma concentration of 142 nM.
in vivo: MK-6096 dose-dependently reduced locomotor activity and significantly increased sleep in rats (3-30 mg/kg) and dogs (0.25 and 0.5 mg/kg). References: | |||||

MK 6096 Dilution Calculator

MK 6096 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3782 mL | 11.8912 mL | 23.7823 mL | 47.5647 mL | 59.4559 mL |
| 5 mM | 0.4756 mL | 2.3782 mL | 4.7565 mL | 9.5129 mL | 11.8912 mL |
| 10 mM | 0.2378 mL | 1.1891 mL | 2.3782 mL | 4.7565 mL | 5.9456 mL |
| 50 mM | 0.0476 mL | 0.2378 mL | 0.4756 mL | 0.9513 mL | 1.1891 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2378 mL | 0.4756 mL | 0.5946 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK 6096
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
Unusual pyrimidine participation: efficient stereoselective synthesis of potent dual orexin receptor antagonist MK-6096.[Pubmed:25365229]
Org Lett. 2014 Nov 21;16(22):5890-3.
An asymmetric synthesis of dual orexin receptor antagonist MK-6096 (1) is described. Key steps for the trans-2,5-disubstituted piperidinyl ether fragment include a biocatalytic transamination, a trans-selective Mukaiyama aldol, and a regioselective pyridyl SNAr process. The pyrimidyl benzoic acid was synthesized via a Negishi coupling and a nitrile hydrolysis. Coupling of the two fragments via a catalytic T3P-mediated amidation completed the synthesis. Unusual behaviors in the hydrolysis of pyrimidyl benzonitrile and the amide coupling of the pyrimidyl benzoic acid are also described.
Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia.[Pubmed:22019562]
Neuropharmacology. 2012 Feb;62(2):978-87.
Orexin (hypocretin) neuropeptides promote wakefulness by signaling through two G-protein coupled receptors, Orexin 1 Receptor (OX(1)R) and Orexin 2 Receptor (OX(2)R). MK-6096 is an orally bioavailable potent and selective reversible antagonist of OX(1)R and OX(2)R currently in clinical development for insomnia. In radioligand binding and functional cell based assays MK-6096 demonstrated potent binding and antagonism of both human OX(1)R and OX(2)R (<3 nM in binding, 11 nM in FLIPR), with no significant off-target activities against a panel of >170 receptors and enzymes. MK-6096 occupies 90% of human OX(2)Rs expressed in transgenic rats at a plasma concentration of 142 nM, and dose-dependently reduced locomotor activity and significantly increased sleep in rats (3-30 mg/kg) and dogs (0.25 and 0.5 mg/kg). DORA-22, an analog of MK-6096, exhibits similar sleep promoting properties that are absent OX(1/2)R double knockouts, demonstrating the mechanism of action and specificity of these effects. These findings with a novel, structurally distinct class of OxR antagonists provide further validation of the orexin pathway as an effective target to promote normal sleep. Comparative analysis of the biochemical and pharmacokinetic properties of these compounds relative to other OXR antagonists provides a basis for understanding the attributes critical for in vivo efficacy. This mechanism is distinct from current standard of care such that MK-6096 represents a novel and selective therapeutic for the treatment of insomnia. This article is part of a Special Issue entitled 'Post-Traumatic Stress Disorder'.
A Phase II Dose-Ranging Study Evaluating the Efficacy and Safety of the Orexin Receptor Antagonist Filorexant (MK-6096) in Patients with Primary Insomnia.[Pubmed:26979830]
Int J Neuropsychopharmacol. 2016 Aug 12;19(8). pii: pyw022.
BACKGROUND: Filorexant (MK-6096) is an orexin receptor antagonist; here, we evaluate the efficacy of filorexant in the treatment of insomnia in adults. METHODS: A double-blind, placebo-controlled, randomized, two 4-week-period, adaptive crossover polysomnography study was conducted at 51 sites worldwide. Patients (18 to <65 years) with insomnia received 1 of 4 doses of oral filorexant (2.5, 5, 10, 20mg) once daily at bedtime during one period and matching placebo in the other period in 1 of 8 possible treatment sequences. Polysomnography was performed on night 1 and end of week 4 of each period. The primary endpoint was sleep efficiency at night 1 and end of week 4. Secondary endpoints included wakefulness after persistent sleep onset and latency to onset of persistent sleep. RESULTS: A total of 324 patients received study treatment, 315 received >/=1 dose of placebo, and 318 >/=1 dose of filorexant (2.5mg, n=79; 5mg, n=78; 10mg, n=80; 20mg, n=81). All filorexant doses (2.5/5/10/20mg) were significantly superior to placebo in improving sleep among patients with insomnia as measured by sleep efficiency and wakefulness after persistent sleep onset on night 1 and end of week 4. The 2 higher filorexant doses (10/20mg) were also significantly more effective than placebo in improving sleep onset as measured by latency to onset of persistent sleep at night 1 and end of week 4. Filorexant was generally well tolerated. CONCLUSIONS: Orexin receptor antagonism by filorexant significantly improved sleep efficiency in nonelderly patients with insomnia. Dose-related improvements in sleep onset and maintenance outcomes were also observed with filorexant.
Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2 -(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties.[Pubmed:22307992]
ChemMedChem. 2012 Mar 5;7(3):415-24, 337.
Insomnia is a common disorder that can be comorbid with other physical and psychological illnesses. Traditional management of insomnia relies on general central nervous system (CNS) suppression using GABA modulators. Many of these agents fail to meet patient needs with respect to sleep onset, maintenance, and next-day residual effects and have issues related to tolerance, memory disturbances, and balance. Orexin neuropeptides are central regulators of wakefulness, and orexin antagonism has been identified as a novel mechanism for treating insomnia with clinical proof of concept. Herein we describe the discovery of a series of alpha-methylpiperidine carboxamide dual orexin 1 and orexin 2 receptor (OX(1) R/OX(2) R) antagonists (DORAs). The design of these molecules was inspired by earlier work from this laboratory in understanding preferred conformational properties for potent orexin receptor binding. Minimization of 1,3-allylic strain interactions was used as a design principle to synthesize 2,5-disubstituted piperidine carboxamides with axially oriented substituents including DORA 28. DORA 28 (MK-6096) has exceptional in vivo activity in preclinical sleep models, and has advanced into phase II clinical trials for the treatment of insomnia.


