U0124CAS# 108923-79-1 |
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108923-79-1 | SDF | Download SDF |
| PubChem ID | 5842955 | Appearance | Powder |
| Formula | C8H10N4S2 | M.Wt | 226.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
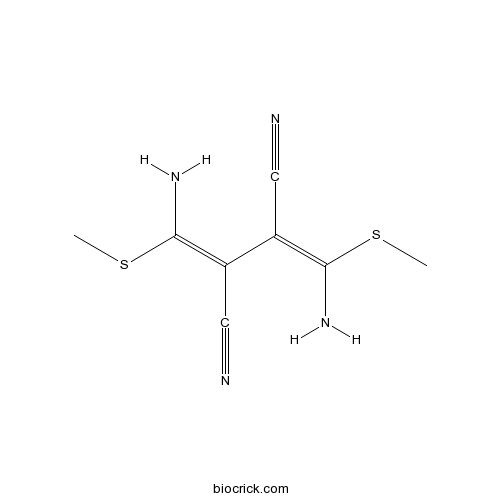
| Chemical Name | (2Z,3Z)-2,3-bis[amino(methylsulfanyl)methylidene]butanedinitrile | ||
| SMILES | CSC(=C(C#N)C(=C(N)SC)C#N)N | ||
| Standard InChIKey | LBQNBMSPTURKGS-KQQUZDAGSA-N | ||
| Standard InChI | InChI=1S/C8H10N4S2/c1-13-7(11)5(3-9)6(4-10)8(12)14-2/h11-12H2,1-2H3/b7-5+,8-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inactive analog of U0126. Used as a negative control. Does not inhibit MEK at concentrations up to 100 μM. |

U0124 Dilution Calculator

U0124 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4187 mL | 22.0936 mL | 44.1872 mL | 88.3744 mL | 110.4679 mL |
| 5 mM | 0.8837 mL | 4.4187 mL | 8.8374 mL | 17.6749 mL | 22.0936 mL |
| 10 mM | 0.4419 mL | 2.2094 mL | 4.4187 mL | 8.8374 mL | 11.0468 mL |
| 50 mM | 0.0884 mL | 0.4419 mL | 0.8837 mL | 1.7675 mL | 2.2094 mL |
| 100 mM | 0.0442 mL | 0.2209 mL | 0.4419 mL | 0.8837 mL | 1.1047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
Induction of neurite outgrowth in PC12 cells treated with temperature-controlled repeated thermal stimulation.[Pubmed:25879210]
PLoS One. 2015 Apr 16;10(4):e0124024.
To promote the functional restoration of the nervous system following injury, it is necessary to provide optimal extracellular signals that can induce neuronal regenerative activities, particularly neurite formation. This study aimed to examine the regulation of neuritogenesis by temperature-controlled repeated thermal stimulation (TRTS) in rat PC12 pheochromocytoma cells, which can be induced by neurotrophic factors to differentiate into neuron-like cells with elongated neurites. A heating plate was used to apply thermal stimulation, and the correlation of culture medium temperature with varying surface temperature of the heating plate was monitored. Plated PC12 cells were exposed to TRTS at two different temperatures via heating plate (preset surface temperature of the heating plate, 39.5 degrees C or 42 degrees C) in growth or differentiating medium for up to 18 h per day. We then measured the extent of growth, neuritogenesis, or acetylcholine esterase (AChE) activity (a neuronal marker). To analyze the mechanisms underlying the effects of TRTS on these cells, we examined changes in intracellular signaling using the following: tropomyosin-related kinase A inhibitor GW441756; p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580; and MAPK/extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor U0126 with its inactive analog, U0124, as a control. While a TRTS of 39.5 degrees C did not decrease the growth rate of cells in the cell growth assay, it did increase the number of neurite-bearing PC12 cells and AChE activity without the addition of other neuritogenesis inducers. Furthermore, U0126, and SB203580, but not U0124 and GW441756, considerably inhibited TRTS-induced neuritogenesis. These results suggest that TRTS can induce neuritogenesis and that participation of both the ERK1/2 and p38 MAPK signaling pathways is required for TRTS-dependent neuritogenesis in PC12 cells. Thus, TRTS may be an effective technique for regenerative neuromedicine.
Insulin-like growth factor-1 receptor-mediated inhibition of A-type K(+) current induces sensory neuronal hyperexcitability through the phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2 pathways, independently of Akt.[Pubmed:24080365]
Endocrinology. 2014 Jan;155(1):168-79.
Although IGF-1 has been implicated in mediating hypersensitivity to pain, the underlying mechanisms remain unclear. We identified a novel functional of the IGF-1 receptor (IGF-1R) in regulating A-type K(+) currents (IA) as well as membrane excitability in small trigeminal ganglion neurons. Our results showed that IGF-1 reversibly decreased IA, whereas the sustained delayed rectifier K(+) current was unaffected. This IGF-1-induced IA decrease was associated with a hyperpolarizing shift in the voltage dependence of inactivation and was blocked by the IGF-1R antagonist PQ-401; an insulin receptor tyrosine kinase inhibitor had no such effect. An small interfering RNA targeting the IGF-1R, or pretreatment of neurons with specific phosphatidylinositol 3-kinase (PI3K) inhibitors abolished the IGF-1-induced IA decrease. Surprisingly, IGF-1-induced effects on IA were not regulated by Akt, a common downstream target of PI3K. The MAPK/ERK kinase inhibitor U0126, but not its inactive analog U0124, as well as the c-Raf-specific inhibitor GW5074, blocked the IGF-1-induced IA response. Analysis of phospho-ERK (p-ERK) showed that IGF-1 significantly activated ERK1/2 whereas p-JNK and p-p38 were unaffected. Moreover, the IGF-1-induced p-ERK1/2 increase was attenuated by PI3K and c-Raf inhibition, but not by Akt blockade. Functionally, we observed a significantly increased action potential firing rate induced by IGF-1; pretreatment with 4-aminopyridine abolished this effect. Taken together, our results indicate that IGF-1 attenuates IA through sequential activation of the PI3K- and c-Raf-dependent ERK1/2 signaling cascade. This occurred via the activation of IGF-1R and might contribute to neuronal hyperexcitability in small trigeminal ganglion neurons.
Induction of neuritogenesis in PC12 cells by a pulsed electromagnetic field via MEK-ERK1/2 signaling.[Pubmed:23318214]
Cell Struct Funct. 2013;38(1):15-20. Epub 2013 Jan 11.
We examined the regulation of neuritogenesis by a pulsed electromagnetic field (PEMF) in rat PC12 pheochromocytoma cells, which can be induced to differentiate into neuron-like cells with elongated neurites by inducers such as nerve growth factor (NGF). Plated PC12 cells were exposed to a single PEMF (central magnetic flux density, 700 mT; frequency, 0.172 Hz) for up to 12 h per day and were then evaluated for extent of neuritogenesis or acetylcholine esterase (AChE) activity. To analyze the mechanism underlying the effect of the PEMF on the cells, its effects on intracellular signaling were examined using the ERK kinase (MEK) inhibitors PD098059 and U0126 (U0124 was used as a negative control for U0126). The number of neurite-bearing PC12 cells and AChE activity increased after PEMF exposure without the addition of other inducers of neuritogenesis. Additionally, PEMF exposure induced sustained activation of ERK1/2 in PC12 cells, but not in NR8383 rat alveolar macrophages. Furthermore, U0126 strongly inhibited PEMF-dependent ERK1/2 activation and neuritogenesis. The PEMF-dependent neuritogenesis was also suppressed by PD098059, but not U0124. These results suggest that PEMF stimulation independently induced neuritogenesis and that activation of MEK-ERK1/2 signaling was induced by a cell-type-dependent mechanism required for PEMF-dependent neuritogenesis in PC12 cells.
Suppression of the cough reflex by inhibition of ERK1/2 activation in the caudal nucleus tractus solitarii of the rabbit.[Pubmed:22301382]
Am J Physiol Regul Integr Comp Physiol. 2012 Apr 15;302(8):R976-83.
The caudal nucleus tractus solitarii (cNTS), the predominant site of termination of cough-related afferents, has been shown to be a site of action of some centrally acting antitussive agents. A role of ERK1/2 has been suggested in acute central processing of nociceptive inputs. Because pain and cough share similar features, we investigated whether ERK1/2 activation could also be involved in the central transduction of tussive inputs. For this purpose, we undertook the present research on pentobarbital sodium-anesthetized, spontaneously breathing rabbits by using microinjections (30-50 nl) of an inhibitor of ERK1/2 activation (U0126) into the cNTS. Bilateral microinjections of 25 mM U0126 caused rapid and reversible reductions in the cough responses induced by both mechanical and chemical (citric acid) stimulation of the tracheobronchial tree. In particular, the cough number and peak abdominal activity decreased. Bilateral microinjections of 50 mM U0126 completely suppressed the cough reflex without affecting the Breuer-Hering inflation reflex, the pulmonary chemoreflex, and the sneeze reflex. These U0126-induced effects were, to a large extent, reversible. Bilateral microinjections of 50 mM U0124, the inactive analog of U0126, at the same cNTS sites had no effect. This is the first study that provides evidence that ERK1/2 activation within the cNTS is required for the mediation of cough reflex responses in the anesthetized rabbit. These results suggest a role for ERK1/2 in the observed effects via nontranscriptional mechanisms, given the short time involved. They also may provide hints for the development of novel antitussive strategies.
Differential adenosine uptake in mixed neuronal/glial or purified glial cultures of avian retinal cells: modulation by adenosine metabolism and the ERK cascade.[Pubmed:21945936]
Biochem Biophys Res Commun. 2011 Oct 14;414(1):175-80.
Adenosine is an important modulator of neuronal survival and differentiation in the CNS. Our previous work showed that nucleoside transporters (NTs) are present in cultures of chick retinal cells, but little is known about the mechanisms regulating adenosine transport in these cultures. Our aim in the present work was to study the participation of the adenosine metabolism as well as the ERK pathway on adenosine uptake in different types of retinal cultures (mixed and purified glial cultures). Kinetic analysis in both cultures revealed that the uptake reached equilibrium after 30 min and presented two components. Incubation of cultures with S-(p-nitrobenzyl)-6-thioinosine (NBTI) or dipyridamole, different inhibitors of equilibrative nucleoside transporters (ENTs), produced a significant and concentration-dependent uptake reduction in both cultures. However, while dipyridamole presented similar maximal inhibitory effects in both cultures (although in different concentrations), the inhibition by NBTI was smaller in glial cultures than in mixed cultures, suggesting the presence of different transporters. Moreover, pre-incubation of [(3)H]-adenosine with adenosine deaminase (ADA) or adenosine kinase (ADK) inhibition with iodotubercidin promoted significant uptake inhibition in both cultures, indicating that the uptake is predominantly for adenosine and not inosine, and that taken up adenosine is preferentially directed to the synthesis of adenine nucleotides. In both cultures, the MEK inhibitors PD98059 or UO126, but not the inactive analog U0124, induced a significant and concentration-dependent uptake decrease. We have not observed any change in adenosine metabolism induced by MEK inhibitors, suggesting that this pathway is mediating a direct effect on NTs. Our results show the expression of different NTs in retinal cells in culture and that the activity of these transporters can be regulated by the ERK pathway or metabolic enzymes such as ADK which are then potential targets for regulation of Ado levels in normal or pathological conditions.
Organ culture of the trigeminal ganglion induces enhanced expression of calcitonin gene-related peptide via activation of extracellular signal-regulated protein kinase 1/2.[Pubmed:20851839]
Cephalalgia. 2011 Jan;31(1):95-105.
BACKGROUND AND OBJECTIVE: Clinical and experimental studies have revealed a central role of calcitonin gene-related peptide (CGRP) in primary headaches. The role of extracellular signal-regulated kinase 1 and 2 (ERK1/2) in neuronal and glial cell expression of CGRP- immunoreactivity (-ir) in rat trigeminal ganglia was studied with an organ culture method. EXPERIMENTAL PROCEDURES: Sections of adult rat trigeminal ganglia were cultured for up to 48 hours, examined with immunohistochemistry and quantitative real-time polymerase chain reaction (PCR) assay. Specific antibodies against CGRP, phosphorylated ERK1/2 (pERK1/2), total ERK1/2 (tERK1/2), phosphorylated p38 (pp38), phosphorylated C-Jun-N-terminal protein kinase (pJNK), pro-calcitonin (pro-CT), CGRP receptor activity modifying protein 1 (RAMP1), glutamine synthetase (GS) and pro-CT were used. To explore molecular mechanisms involved in the organ culture-induced CGRP-ir in neurons and glial cells, the effects of the MEK/ERK1/2 inhibitor U0126, its inactive analogue U0124, the p38 inhibitor SB203580 and the JNK inhibitor SP600125 were studied. RESULTS: In fresh ganglia, small- and medium-sized neurons were CGRP-ir while some larger neurons displayed RAMP1-ir. Glial cells were negative to both. After organ culture, neurons showed enhanced CGRP- and RAMP1-ir. In addition, some glial cells were RAMP1- and CGRP-ir. Isolated glial cells and neurons were found to contain CGRP mRNA, and showed pro-CT-ir, suggestive of local formation of CGRP. Neurons and glial cells showed enhanced pERK1/2-ir already after two hours of organ culture and this remained elevated for 48 hours. There was transient pJNK-ir in neurons at two hours, while pp38-ir was not altered. U0126 reduced the enhanced pERK1/2-ir, while U0124 had no such effect; the CGRP-ir in neurons and glial cells was reduced at 48 hours and in parallel the CGRP mRNA expression was lower at 24 hours. CONCLUSION: We suggest that in conditions of elevated CGRP expression, inhibition of ERK1/2 might be an option for novel treatment.
Leptin and ObRa/MEK signalling in mouse oocyte maturation and preimplantation embryo development.[Pubmed:19712552]
Reprod Biomed Online. 2009 Aug;19(2):181-90.
Recent studies indicate that LH stimulates production of ovarian paracrine factors that induce meiosis of the oocyte. DNA microarray analyses of ovarian transcripts were performed in mice and major increases of a short isoform of leptin receptor, ObRa, were identified by the preovulatory LH/human chorionic gonadotrophin (HCG) surge. In oocytes, the level of ObRa transcripts was increased shortly after HCG stimulation, whereas the level of ObRb transcripts was not changed. Leptin was produced by cumulus, granulosa, theca and interstitial cells of ovaries and its transcript level was not regulated during gonadotrophin treatment. Treatment with leptin promoted germinal vesicle breakdown (GVBD) in oocytes within preovulatory follicles, and enhance first polar body extrusion in both cumulus-oocyte complexes and denuded oocytes. The leptin-promoted GVBD and first polar body extrusion were blocked by a mitogen-activated protein kinase extracellular signal regulated kinase kinases (MEK)1/2 inhibitor, U0126, but not its inactive analogue U0124. Furthermore, leptin promoted fertilization of oocytes and the in-vitro development of zygotes to preimplantation embryos. These findings suggest paracrine roles of leptin in the enhancement of nuclear maturation of oocytes through MEK1/2 signalling, and in the promotion of cytoplasmic maturation essential for successful oocyte development to the preimplantation embryos.
Carbachol induces p70S6K1 activation through an ERK-dependent but Akt-independent pathway in human colonic epithelial cells.[Pubmed:19615971]
Biochem Biophys Res Commun. 2009 Sep 25;387(3):521-4.
Stimulation of human colonic epithelial T84 cells with the muscarinic receptor agonist carbachol, a stable analog of acetylcholine, induced Akt, p70S6K1 and ERK activation. Treatment of T84 cells with the selective inhibitor of EGF receptor (EGFR) tyrosine kinase AG1478 abrogated Akt phosphorylation on Ser(473) induced by either carbachol or EGF, indicating that carbachol-induced Akt activation is mediated through EGFR transactivation. Surprisingly, AG1478 did not suppress p70S6K1 phosphorylation on Thr(389) in response to carbachol, indicating the G protein-coupled receptor (GPCR) stimulation induces p70S6K1 activation, at least in part, via an Akt-independent pathway. In contrast, treatment with the selective MEK inhibitor U0126 (but not with the inactive analog U0124) inhibited carbachol-induced p70S6K1 activation, indicating that the MEK/ERK/RSK pathway plays a critical role in p70S6K1 activation in GPCR-stimulated T84 cells. These findings imply that GPCR activation induces p70S6K1 via ERK rather than through the canonical PI 3-kinase/Akt/TSC/mTORC1 pathway in T84 colon carcinoma cells.
Heme oxygenase activity and hemoglobin neurotoxicity are attenuated by inhibitors of the MEK/ERK pathway.[Pubmed:19371583]
Neuropharmacology. 2009 Apr;56(5):922-8.
Hemoglobin breakdown produces an iron-dependent neuronal injury after experimental CNS hemorrhage that may be attenuated by heme oxygenase (HO) inhibitors. The HO enzymes are phosphoproteins that are activated by phosphorylation in vitro. While testing the effect of kinase inhibitors in cortical cell cultures, we observed that HO activity was consistently decreased by the MEK inhibitor U0126. The present study tested the hypothesis that MEK/ERK pathway inhibitors reduce HO activity and neuronal vulnerability to hemoglobin. The MEK inhibitors U0126 and SL327 and the ERK inhibitor FR180204 reduced baseline culture HO activity by 35-50%, without altering the activity of recombinant HO-1 or HO-2; negative control compounds U0124 and FR180289 had no effect. Hemoglobin exposure for 16h produced widespread neuronal injury, manifested by release of 59.2+/-7.8% of neuronal lactate dehydrogenase and a twelve-fold increase in malondialdehyde; kinase inhibitors were highly protective. HO-1 induction after hemoglobin treatment was also decreased by U0126, SL327, and FR180204. These results suggest that reduction in HO activity may contribute to the protective effect of MEK and ERK inhibitors against heme-mediated neuronal injury.
Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia.[Pubmed:19210515]
Cephalalgia. 2009 May;29(5):520-31.
The mitogen-activated protein kinase, extracellular signal-regulated kinase (ERK), is activated in experimental models of chronic pain, and is also activated by oestrogen. We used an established model of inflammatory trigeminal pain, injection of Complete Freund's Adjuvant (CFA) into the masseter muscle, to determine whether ERK activation may play a role in hormone-related trigeminal pain disorders. We measured withdrawal responses to stimulation of the masseter (V3, primary allodynia) and whisker pad (V2, secondary allodynia) using graded monofilaments. Oestrogen treatment in the presence of inflammation increased withdrawal response to stimulation of both masseter and whisker pad compared with inflammation alone, indicating an additive effect of inflammation and oestrogen on both primary and secondary allodynia. We examined ERK activation in trigeminal ganglia from each treatment group using western blot and immunohistochemistry. Both masseter inflammation and oestrogen treatment increased ERK activation, and combined treatment had an additive effect. Both masseter inflammation and oestrogen increased the percentage of pERK immunoreactive neurons in divisions 1 and 2 (V1/2), and combined treatment increased pERK immunoreactivity in V1/2 compared with inflammation alone. We stereotactically administered ERK antagonist U0126, or inactive control U0124, to the trigeminal ganglion of CFA+E2-treated rats. U0126 decreased withdrawal responses to mechanical stimulation of the whisker pad compared with U0124-treated rats. Because the secondary allodynia in V2 after inflammation in V3 was reduced by antagonizing ERK activation in the periphery, these data suggest a peripheral component to secondary allodynia mediated through ERK activation.
Partial dissociation of molecular and behavioral measures of song habituation in adult zebra finches.[Pubmed:19125865]
Genes Brain Behav. 2008 Oct;7(7):802-9.
Initial playback of recorded birdsong triggers a number of responses in zebra finches, including overt listening behavior and ERK pathway-dependent activation of zenk gene transcription in the auditory lobule of the forebrain. Repetition of one song stimulus leads to persistent habituation of these responses, as measured by subsequent presentations 1 day later. In this study, we examined the causal relationships between behavioral and molecular (ERK/zenk) habituation. In a within-subject comparison, we found a strong correlation with the level of prior training for both responses (duration of behavioral listening and magnitude of zenk expression), but little correlation between these responses for birds within the same treatment group. We then tested the hypothesis that ERK/zenk activation during training is necessary for the development of habituation measured 1 day later. Cannula-directed infusion of a pharmacological inhibitor of ERK activation (U0126) immediately before training blocked the development of habituation of the zenk gene response. However, measurement of the effect on behavioral habituation was confounded because birds that were infused with a non-active drug analogue (U0124) showed a decreased response 1 day later, even to novel songs. We conclude that the behavioral response to song stimulation is strongly influenced by factors other than song familiarity, whereas the zenk response in the forebrain may be a more accurate indicator of actual experience hearing a particular song.
PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior.[Pubmed:18631385]
Mol Pain. 2008 Jul 16;4:26.
The laterocapsular division of the central nucleus of the amygdala (CeLC) has emerged as an important site of pain-related plasticity and pain modulation. Glutamate and neuropeptide receptors in the CeLC contribute to synaptic and behavioral changes in the arthritis pain model, but the intracellular signaling pathways remain to be determined. This study addressed the role of PKA, PKC, and ERK in the CeLC. Adult male Sprague-Dawley rats were used in all experiments. Whole-cell patch-clamp recordings of CeLC neurons were made in brain slices from normal rats and from rats with a kaolin/carrageenan-induced monoarthritis in the knee (6 h postinduction). Membrane-permeable inhibitors of PKA (KT5720, 1 microM; cAMPS-Rp, 10 microM) and ERK (U0126, 1 microM) activation inhibited synaptic plasticity in slices from arthritic rats but had no effect on normal transmission in control slices. A PKC inhibitor (GF109203x, 1 microM) and an inactive structural analogue of U0126 (U0124, 1 microM) had no effect. The NMDA receptor-mediated synaptic component was inhibited by KT5720 or U0126; their combined application had additive effects. U0126 did not inhibit synaptic facilitation by forskolin-induced PKA-activation. Administration of KT5720 (100 microM, concentration in microdialysis probe) or U0126 (100 microM) into the CeLC, but not striatum (placement control), inhibited audible and ultrasonic vocalizations and spinal reflexes of arthritic rats but had no effect in normal animals. GF109203x (100 microM) and U0124 (100 microM) did not affect pain behavior. The data suggest that in the amygdala PKA and ERK, but not PKC, contribute to pain-related synaptic facilitation and behavior by increasing NMDA receptor function through independent signaling pathways.
Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases.[Pubmed:17662043]
Immunology. 2008 Feb;123(2):171-80.
Human neutrophil migratory responses to Toll-like receptor (TLR) agonists were studied using videomicroscopy. When challenged with lipopolysaccharide (LPS, TLR4 agonist) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3) -lysine (P3CSK4, TLR2 agonist), neutrophils displayed enhanced motility, which was found to reflect increased random migration but not directed migration (chemotaxis). Enhanced neutrophil motility was detected within 10 min after stimulation with LPS or P3CSK4, and was sustained for more than 80 min. Stimulation of neutrophils with LPS or P3CSK4 resulted in the activation of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK), which preceded neutrophil migration. TLR-mediated neutrophil migration was strongly suppressed by pretreatment of cells with U0126 (MAPK/ERK kinase inhibitor) but not with U0124 (an inactive analogue of U0126) or SB203580 (a p38 MAPK inhibitor), and was almost completely abolished by pretreatment of cells with U0126 and SB203580 in combination. Randomly migrating neutrophils in response to LPS or P3CSK4 displayed directed migration when further challenged with gradient concentrations of N-formyl-methionyl-leucyl-phenylalanine (FMLP) or platelet-activating factor (PAF). These findings indicate that TLR agonists stimulate human neutrophil migration via the activation of ERK and p38 MAPK, and FMLP- or PAF-induced neutrophil chemotaxis is not affected by the pre-exposure of cells to TLR agonists.
A mitogen-activated protein kinase signals to programmed cell death induced by self-incompatibility in Papaver pollen.[Pubmed:17660353]
Plant Physiol. 2007 Sep;145(1):236-45.
Self-incompatibility (SI) in higher plants is an important mechanism to prevent inbreeding and involves specific rejection of incompatible ("self") pollen. In field poppy (Papaver rhoeas), S proteins encoded by the stigma component of the S-locus interact with incompatible pollen, resulting in cessation of tip growth. This "self" interaction triggers a Ca(2+)-dependent signaling network, involving programmed cell death (PCD). We previously identified p56, a mitogen-activated protein kinase (MAPK) that is activated during the SI response in incompatible pollen. Here, we show that p56 cross-reacts with AtMPK3, but not with AtMPK4 or salicylic acid-induced protein kinase antibodies. We provide good evidence that a MAPK is involved in initiation of SI-induced PCD in incompatible pollen. SI rapidly reduces pollen viability and the MAPK cascade inhibitor U0126, which prevents the SI-induced activation of p56 in incompatible pollen, "rescues" incompatible pollen, while its negative analog, U0124, does not. This strongly implicates the involvement of a MAPK in SI-mediated loss of pollen viability and cell death. SI also stimulates caspase-3-like (DEVDase) activity and later DNA fragmentation. Both these markers of PCD are significantly reduced by pretreatment with U0126, implicating the involvement of a MAPK in signaling during early PCD. As p56 appears to be the only MAPK activated by SI, our studies imply that p56 could be the MAPK involved in mediating SI-induced PCD.
Neural induction in the absence of organizer in salamanders is mediated by MAPK.[Pubmed:17540356]
Dev Biol. 2007 Jul 15;307(2):282-9.
Research on the mechanisms of embryonic induction had a great setback in the 1940s when Barth discovered and Holtfreter confirmed that ectoderm of Ambystoma maculatum salamander embryos could form brain tissue when cultured in a simple saline solution. We have revisited this classical experiment and found that when cultured animal cap ectoderm attaches to a glass substratum, it can self-organize to form complex organs such as brain vesicles, eyes, lens and olfactory placodes. Only anterior neural organs were generated. Under these culture conditions ERK became diphosphorylated, indicating a sustained activation of the Ras/MAPK pathway. Using sand particles as an example of a heterologous neural inducer similar results were obtained. Addition of U0126, a specific antagonist of MEK, the enzyme that phosphorylates ERK/MAPK, inhibited neural differentiation. The closely related control compound U0124 had no effect. We conclude that neural induction in the absence of organizer in A. maculatum requires Ras/MAPK-activation. These findings provide a molecular explanation for the activity of heterologous neural inducers that dominated thinking in amphibian experimental embryology for many decades.
Concerted transcriptional activation of the low density lipoprotein receptor gene by insulin and luteinizing hormone in cultured porcine granulosa-luteal cells: possible convergence of protein kinase a, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase signaling pathways.[Pubmed:11416012]
Endocrinology. 2001 Jul;142(7):2921-8.
Insulin and insulin-like growth factor I (IGF-I) can amplify gonadotropin-stimulated steroidogenesis by augmenting the expression of key sterol regulatory genes in ovarian cells, viz. low density lipoprotein (LDL) receptor, steroidogenic acute regulatory protein, and P450 cholesterol side-chain cleavage enzyme (CYP11A). The mechanisms underlying the foregoing bihormonal interactions are not known. Accordingly, in relation to the LDL receptor gene, the present study tests the hypothesis that insulin/IGF-I and LH can act via concerted transcriptional control of promoter expression. To this end, we transiently transfected primary monolayer cultures of porcine granulosa-luteal cells with a reporter vector containing the putative 5'-upstream full-length (pLDLR1076/luc) regulatory region (-1076 to +11 bp) of the homologous LDL receptor gene driving firefly luciferase in the presence or absence of insulin (or IGF-I) and/or LH (each 100 ng/ml). Combined exposure to LH and insulin (or IGF-I) stimulated LDL receptor transcriptional activity maximally at 4 h by 8- to 20-fold, as normalized by coexpression of Renilla luciferase. Further analysis of multiple 5'-nested deletional constructs of the LDL receptor gene promoter showed that deletion of -139 bp upstream of the transcriptional start site virtually abolished basal expression and promoter responsiveness to LH and insulin/IGF-I. In contrast, full basal activity and 60-80% of maximal monohormonal and bihormonal drive were retained by the -255 to +11 bp fragment. As LDL receptor gene expression in other tissues is negatively regulated by the abundance of intracellular free cholesterol, we assessed the impact of concomitant pretreatment of granulosa-luteal cells with an exogenous soluble sterol (25-hydroxycholesterol, 1 and 10 microM). Excess sterol markedly (50-70%) attenuated bihormonally and, in lesser measure, LH-stimulated and basal LDL receptor promoter expression, thus affirming a feedback-sensitive sterol-repressive region in this gene. Non-LH receptor-dependent agonists of protein kinase A (PKA), 8-bromo-cAMP (1 mM), and forskolin (10 microM) with or without insulin/IGF-I costimulation likewise augmented LDL receptor promoter expression with similar strong dependency on the -255 to -139 bp 5'-upstream region. To assess more specific PKA-dependent mediation of LH's contribution to combined hormonal drive, the LDL receptor (-1076 to +11 bp) reporter plasmid was cotransfected with a full-sequence rabbit muscle protein kinase inhibitor (PKI) minigene driven constitutively by a Rous sarcoma virus promoter. Expression of the latter PKA antagonist blocked transcriptional stimulation by LH alone as well as that by LH combined with insulin (or IGF-I) by 70-85% without reducing basal transcriptional activity. Transfection of a mutant inactive (Arg to Gly) Rous sarcoma virus/PKI gene confirmed the specificity of the PKI effect. To investigate the convergent role of the insulin/IGF-I effector pathway mediating bihormonal stimulation of LDL receptor promoter expression, transfected granulosa-luteal cells were pretreated for 30 min with two specific inhibitors of phophatidylinositol 3-kinase, wortmannin (100 nM) and LY 294002 (10 microM), or of mitogen-activated protein kinase kinase, PD 98059 (50 microM), U0126 (10 microM), or the latter's inactive derivative, U0124 (10 microM). Both classes of antagonists impeded the ability of insulin or IGF-I to enhance LH-stimulated LDL receptor promoter expression by 60-80%. In conclusion, the present analyses indicate that LH and insulin (or IGF-I) can up-regulate LDL receptor transcriptional activity supraadditively in porcine granulosa-luteal cells 1) via one or more agonistic cis-acting DNA regions located between -255 and -139 bp 5'- upstream of the transcriptional start site, 2) without abrogating sterol-sensitive repressive of this promoter, and 3) by way of intracellular mechanisms that include the PKA, phophatidylinositol 3-kinase, and mitogen-activated protein kinase signaling pathways.
Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway.[Pubmed:10990531]
J Physiol. 2000 Sep 15;527 Pt 3:433-44.
The small G-protein Ras, a critical component in the signalling pathways regulating cell growth, is involved in the tonic upregulation of voltage-dependent calcium channels (VDCCs) in rat sensory neurones. To investigate which downstream effector(s) of Ras is involved in this process, a series of Ras mutant cDNAs were co-expressed with green fluorescent protein (GFP) in primary cultured rat dorsal root ganglion neurones (DRGs). Constitutively active V12Ras (glycine 12 to valine) markedly increased basal calcium current density by 41 % compared with control cells (GFP alone). In contrast, a farnesylation-defective mutant, V12S186Ras (cysteine 186 to serine; activates no downstream effectors), significantly reduced calcium current density by 47 %. Ras effector region mutants V12C40 (tyrosine 40 to cysteine; activates the p110 alpha-subunit of phosphatidylinositol 3-kinase) and V12G37 (glutamic acid 37 to glycine; activates Ral guanine nucleotide dissociation stimulator) had no significant effect on VDCC current. However, V12S35Ras (threonine 35 to serine; activates Raf-1 and the mitogen-activated protein kinase (MAPK) pathway) markedly increased basal calcium current density by 67 %, suggesting that Raf-1 activation is sufficient for Ras enhancement of calcium current in these cells. Raf-1 activates MEK (MAPK kinase) in the MAPK pathway, and the MEK inhibitor U0126 reduced calcium current by 45 % after 10-15 min, whereas the inactive analogue U0124 had no effect. This rapid time course for MEK inhibition suggests direct modulation of VDCCs via the Ras-MAPK pathway rather than gene expression-mediated effects. The relative proportions of omega-conotoxin GVIA- and nicardipine-sensitive N- ( approximately 40 %) and L- ( approximately 40 %) type currents were unaffected by either V12S35Ras expression or U0126 pre-treatment, suggesting that all components of calcium current in DRGs, are enhanced via this pathway.
Identification of a novel inhibitor of mitogen-activated protein kinase kinase.[Pubmed:9660836]
J Biol Chem. 1998 Jul 17;273(29):18623-32.
The compound U0126 (1,4-diamino-2,3-dicyano-1, 4-bis[2-aminophenylthio]butadiene) was identified as an inhibitor of AP-1 transactivation in a cell-based reporter assay. U0126 was also shown to inhibit endogenous promoters containing AP-1 response elements but did not affect genes lacking an AP-1 response element in their promoters. These effects of U0126 result from direct inhibition of the mitogen-activated protein kinase kinase family members, MEK-1 and MEK-2. Inhibition is selective for MEK-1 and -2, as U0126 shows little, if any, effect on the kinase activities of protein kinase C, Abl, Raf, MEKK, ERK, JNK, MKK-3, MKK-4/SEK, MKK-6, Cdk2, or Cdk4. Comparative kinetic analysis of U0126 and the MEK inhibitor PD098059 (Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J., and Saltiel, A. R. (1995) Proc. Natl. Acad. Sci U. S. A. 92, 7686-7689) demonstrates that U0126 and PD098059 are noncompetitive inhibitors with respect to both MEK substrates, ATP and ERK. We further demonstrate that the two compounds bind to deltaN3-S218E/S222D MEK in a mutually exclusive fashion, suggesting that they may share a common or overlapping binding site(s). Quantitative evaluation of the steady state kinetics of MEK inhibition by these compounds reveals that U0126 has approximately 100-fold higher affinity for deltaN3-S218E/S222D MEK than does PD098059. We further tested the effects of these compounds on the activity of wild type MEK isolated after activation from stimulated cells. Surprisingly, we observe a significant diminution in affinity of both compounds for wild type MEK as compared with the deltaN3-S218E/S222D mutant enzyme. These results suggest that the affinity of both compounds is mediated by subtle conformational differences between the two activated MEK forms. The MEK affinity of U0126, its selectivity for MEK over other kinases, and its cellular efficacy suggest that this compound will serve as a powerful tool for in vitro and cellular investigations of mitogen-activated protein kinase-mediated signal transduction.


