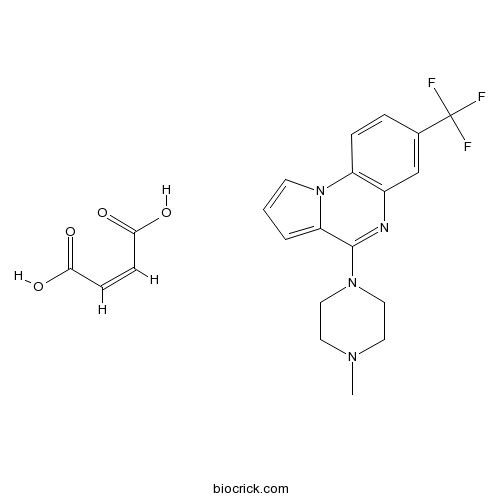
CGS 12066B dimaleate5-HT1B agonist CAS# 109028-10-6 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109028-10-6 | SDF | Download SDF |
| PubChem ID | 6438352 | Appearance | Powder |
| Formula | C21H21F3N4O4 | M.Wt | 450.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water and to 40 mM in DMSO | ||
| Chemical Name | (Z)-but-2-enedioic acid;4-(4-methylpiperazin-1-yl)-7-(trifluoromethyl)pyrrolo[1,2-a]quinoxaline | ||
| SMILES | CN1CCN(CC1)C2=NC3=C(C=CC(=C3)C(F)(F)F)N4C2=CC=C4.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | ZBPAHEUAJMCLRD-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C17H17F3N4.C4H4O4/c1-22-7-9-23(10-8-22)16-15-3-2-6-24(15)14-5-4-12(17(18,19)20)11-13(14)21-16;5-3(6)1-2-4(7)8/h2-6,11H,7-10H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT1B full agonist, 10-fold selective over 5-HT1A and 1000-fold selective over 5-HT2C receptors. Centrally active following systemic administration. |

CGS 12066B dimaleate Dilution Calculator

CGS 12066B dimaleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2202 mL | 11.1012 mL | 22.2025 mL | 44.405 mL | 55.5062 mL |
| 5 mM | 0.444 mL | 2.2202 mL | 4.4405 mL | 8.881 mL | 11.1012 mL |
| 10 mM | 0.222 mL | 1.1101 mL | 2.2202 mL | 4.4405 mL | 5.5506 mL |
| 50 mM | 0.0444 mL | 0.222 mL | 0.444 mL | 0.8881 mL | 1.1101 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.222 mL | 0.444 mL | 0.5551 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in an operant procedure in rats.[Pubmed:10837852]
Pharmacol Biochem Behav. 2000 May;66(1):129-36.
Recent evidence suggests that 5-HT(1B) receptor activation modifies ethanol's reinforcing, intoxicating and discriminative stimulus effects. The present study further explored the role played by 5-HT(1A/1B) receptors by examining their influence on oral ethanol self-administration. Male Wistar rats were trained on an FR 4 schedule to obtain a reinforcer of 0.1 12% w/v ethanol solution. Once responding was stable, the effect of the 5-HT(1A/1B) agonist RU24969 alone and in combination with the 5-HT(1B) antagonist GR127935 or the 5-HT(1A) antagonists (+) WAY100135 and (+) WAY100635 was assessed. The effect of RU24969 on ethanol's pharmacokinetic profile and on operant oral saline self-administration was also examined to assess if alterations in oral ethanol self-administration were due to nonspecific effects on level pressing. For comparison, we examined the effect of another 5-HT(1A/1B) agonist, CGS12066B, on oral ethanol self-administration. Both RU24969 (0.1 to 1 mg/kg) and CGS12066B (0.1 to 1 mg/kg) significantly suppressed oral ethanol self-administration. Administration of GR127935 (1 mg/kg), significantly reversed the effects elicited by RU24969, whereas neither WAY100635 (1 mg/kg) nor (+)WAY100135 (1 mg/kg) had any effect. The effects of lower doses of RU24969 on oral ethanol self-administration were selective as oral saline self-administration and blood ethanol levels were not altered by these doses. These data demonstrate that 5-HT(1B) receptor activation suppresses oral ethanol self-administration. These studies provide further evidence that 5-HT(1B) receptors play a modulatory role in ethanol's behavioral effects.
Interaction of arylpiperazines with 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors: do discriminatory 5-HT1B receptor ligands exist?[Pubmed:2770889]
Naunyn Schmiedebergs Arch Pharmacol. 1989 Jun;339(6):675-83.
The effects of several putative 5-HT1 receptor-subtype selective ligands were investigated in biochemical models for 5-HT1A, 5-HT1B, and 5-HT1D receptors (inhibition of forskolin-stimulated adenylate cyclase activity in calf hippocampus, rat and calf substantia nigra, respectively) and 5-HT1C receptors (stimulation of inositol phosphates production in pig choroid plexus). Following compounds were studied: 5-HT (5-hydroxytryptamine), TFMPP (1-(m-trifluoromethylphenyl)piperazine), mCPP (1-(m-chlorophenyl)piperazine), CGS 12066 (7-trifluoromethyl-4-(4-methyl-1-piperazinyl)-pyrrolo[1,2-a] quinoxaline 1), isamoltane (CGP 361A, 1-(2-(1-pyrrolyl)-phenoxy)-3-isopropylamino-2-propranol), quipazine, 1-NP (1-(1-naphthyl)piperazine), and PAPP (LY165163, 1-[2-(4-aminophenyl)ethyl]-4-(3-trifluoromethylphenyl)- piperazine). Among reported 5-HT1B receptor selective drugs, TFMPP had similar potency at 5-HT1A, 5-HT1B and 5-HT1C receptors, mCPP did not separate between 5-HT1B and 5-HT1C receptors, CGS 12066 was equipotent at 5-HT1B and 5-HT1D receptors, and isamoltane was only slightly 5-HT1B versus 5-HT1A selective. Quipazine showed equal potency at 5-HT1B and 5-HT1C receptors and 1-NP did not discriminate between the four receptor subtypes. PAPP described as 5-HT1A receptor selective, was equally potent at 5-HT1A and 5-HT1D receptors. The potencies determined in second messenger studies were in good agreement with the affinity values determined in radioligand binding studies. Thus 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors have different pharmacological profiles as predicted from radioligand binding studies. Despite claims to the contrary, none of the tested compounds had actual selectivity for a given 5-HT1 receptor subtype.(ABSTRACT TRUNCATED AT 250 WORDS)
Biochemical and pharmacological characterization of CGS 12066B, a selective serotonin-1B agonist.[Pubmed:3496228]
Eur J Pharmacol. 1987 Apr 7;136(1):1-9.
CGS 12066B is a novel pyrroloquinoxaline with selectivity for the serotonin-1B (5HT1B) recognition site as assessed by binding, biochemical and electrophysiological studies. The compound had an IC50 value of 51 nM at the 5HT1B recognition site as determined using the binding of [3H]5HT in the presence of 1 microM spiperone. At the 5HT1A receptor the compound had an IC50 value of 876 nM, providing a 5HT1A/5HT1B ratio of 17 in contrast to the putative 5HT1B selective agent trifluoromethylphenylpiperazine (TFMPP) which had a corresponding ratio of 3.6. The compound had minimal affinity for alpha 1-, alpha 2- and beta-adrenoceptors and for dopamine D-1 and D-2 receptors. CGS 12066B, in contrast to TFMPP, which was inactive, was found to inhibit dorsal raphe cell firing with an ED50 value of 358 nmol/kg i.v. The corresponding values for the 5HT1A selective agonists 8-OH-DPAT and ipsapirone were 1.3 and 33 nmol/kg. CGS 12066B was also effective in decreasing rat brain 5-HTP concentrations and inhibiting in vitro 5HT release. The data obtained indicate that CGS 12066B is a reasonably active 5HT1B site agonist, which due to its selectivity as compared to compounds such as TFMPP, will be a useful tool for evaluating the physiological role of such receptors in the mammalian CNS.


