PP242MTOR inhibitor, selective and ATP-competitive CAS# 1092351-67-1 |
- WYE-687
Catalog No.:BCC4604
CAS No.:1062161-90-3
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
- INK 128 (MLN0128)
Catalog No.:BCC3880
CAS No.:1224844-38-5
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092351-67-1 | SDF | Download SDF |
| PubChem ID | 25243800 | Appearance | Powder |
| Formula | C16H16N6O | M.Wt | 308.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (162.16 mM; Need ultrasonic) | ||
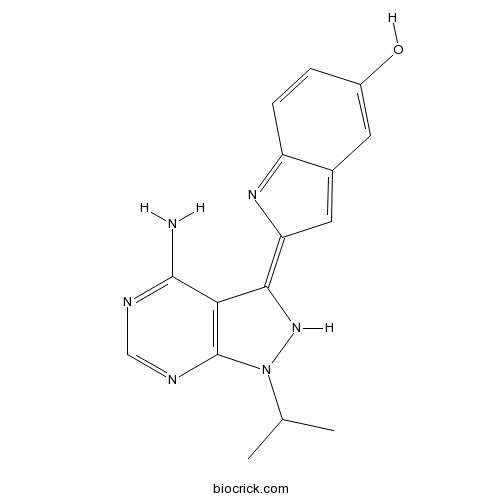
| Chemical Name | (2E)-2-(4-amino-1-propan-2-yl-2H-pyrazolo[3,4-d]pyrimidin-3-ylidene)indol-5-ol | ||
| SMILES | CC(C)N1C2=C(C(=C3C=C4C=C(C=CC4=N3)O)N1)C(=NC=N2)N | ||
| Standard InChIKey | MTICIDWIKCANDD-WYMLVPIESA-N | ||
| Standard InChI | InChI=1S/C16H16N6O/c1-8(2)22-16-13(15(17)18-7-19-16)14(21-22)12-6-9-5-10(23)3-4-11(9)20-12/h3-8,21,23H,1-2H3,(H2,17,18,19)/b14-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ATP-competitive mTORC1/mTORC2 inhibitor (IC50 = 8 nM). Displays selectivity for mTOR over other PI 3K family kinases (IC50 values are 0.102, 0.408, 1.27, 1.96 and 2.2 μM for p110γ, DNA-PK, p110δ, p110α and p110β respectively) and 215 further kinases. Displays modest inhibition of PKCα, JAK2, PKCβI, PKCβII and RET (IC50 values are 0.049, 0.110, 0.185, 0.198 and 0.224 μM respectively). Inhibits both S6K and 4EBP1 phosphorylation; activity causes a decrease in cap-dependent protein translation. Also triggers downregulation of cFLIPS and augments TRAIL-induced apoptosis of cancer cells. |

PP242 Dilution Calculator

PP242 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2431 mL | 16.2153 mL | 32.4307 mL | 64.8614 mL | 81.0767 mL |
| 5 mM | 0.6486 mL | 3.2431 mL | 6.4861 mL | 12.9723 mL | 16.2153 mL |
| 10 mM | 0.3243 mL | 1.6215 mL | 3.2431 mL | 6.4861 mL | 8.1077 mL |
| 50 mM | 0.0649 mL | 0.3243 mL | 0.6486 mL | 1.2972 mL | 1.6215 mL |
| 100 mM | 0.0324 mL | 0.1622 mL | 0.3243 mL | 0.6486 mL | 0.8108 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PP242, a novel potent and selective mTOR inhibitor, can inhibit the active site of mTOR kinase in both mTORC1 and mTORC2 with IC50 of 8 nM.
The mammalian target of rapamycin (mTOR), a serine-threonine kinase, is present in two protein complexes, mTORC1 and mTORC2, that have distinct subunit composition, substrates and mechanisms of activation.
Treatment of PP242 was shown to lead to the death of certain mouse and human leukemia cells [1]. In primary AML cells and CD34+ progenitor cells, PP242 can inhibit the activity of mTOR and their downstream targets, therefore inducing cell apoptosis [2].
The component has also been used to study the role of COX-2 in vivo. In models of acute leukemia harboring the Philadelphia chromosome (Ph) translocation, PP242 can delay the onset of leukemia and augment the effects of front-line tyrosine kinase inhibitors more effectively, thereby suppressing the expansion of leukemia [1]. In addition, PP242 was shown to suppresse leukemia progression in a murine leukemia model which was driven by mutated FLT3 with constitutive activation of mTOR [2].
References:
1.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med 2010,16:205-213.
2.Zeng Z, Shi YX, Tsao T, Qiu Y, Kornblau SM, Baggerly KA, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood 2012,120:2679-2689.
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
- (3S,4R)-Tofacitinib
Catalog No.:BCC4267
CAS No.:1092578-48-7
Combination of cetuximab and PP242 synergistically suppress the progression of wild-type KRAS colorectal carcinoma.[Pubmed:26586952]
Onco Targets Ther. 2015 Nov 2;8:3185-92.
Mammalian target of rapamycin (mTOR) has been shown to be overactive in human colorectal cancer, but the first-generation mTOR inhibitor, rapamycin, has failed to show clinical efficacy against colorectal cancer. On the other hand, although the second-generation mTOR inhibitor, PP242, has exerted substantial efficacy, it was revealed that independent inhibition by PP242 was transient, which could lead to positive-feedback loop to EGFR. Using wild-type KRAS colorectal cancer cells as models, we investigate the treatment efficacy of a widely used anti-EGFR monoclonal antibody, cetuximab, and PP242, alone or in combination in vitro and in vivo. Results of cell viability assays confirmed the synergistic inhibitory effect of PP242 and cetuximab on the survival of Caco-2 and HT-29 cells. Moreover, the ability of cancer-cell invasion and proliferation was also significantly inhibited by the combination therapy when compared with cetuximab or PP242 alone. Interestingly, the percentage of CD44-positive cancer cells was substantially decreased by the combination therapy in comparison with PP242 alone through fluorescence-activated cell sorting. The growth of cancer stem-like cell spheres in vitro was also maximally inhibited by combination therapy, in terms of either diameter or number. More importantly, the efficacy of combination therapy was more prominent than either drug alone in established tumor xenografts. These findings supported the potential use of combination therapy of PP242 and cetuximab against wild-type KRAS colorectal carcinomas.
mTORC2 modulates feedback regulation of p38 MAPK activity via DUSP10/MKP5 to confer differential responses to PP242 in glioblastoma.[Pubmed:25568665]
Genes Cancer. 2014 Nov;5(11-12):393-406.
Dual-specificity phosphatases (DUSPs) dephosphorylate MAP kinases (MAPKs) resulting in their inactivation. Activation of MAPK signaling leads to enhanced DUSP expression, thus establishing feedback regulation of the MAPK pathway. The DUSPs are subject to regulation at the post-translational level via phosphorylation resulting in alterations of protein stability. Here we report that mTORC2 function leads to stabilization of the p38 MAPK phosphatase, DUSP10, thereby inhibiting p38 activity. We demonstrate that mTORC2 binds DUSP10 and phosphorylates DUSP10 on serine residues 224 and 230. These phosphorylation events block DUSP10 turnover resulting in inactivation of p38 signaling. We further show that insulin-stimulated PI3K/mTORC2 signaling regulates DUSP10 stability and p38 activity. Importantly, knockdown of DUSP10 or ectopic overexpression of nonphosphorylatable or phosphomimetic DUSP10 mutants was sufficient to confer differential mTOR kinase inhibitor responses to GBM cells in vitro and in murine xenografts. Finally, DUSP10 was shown to be overexpressed in a significant number of GBM patients. These data demonstrate the ability of the mTORC2 pathway to exert regulatory effects on the DUSP10/p38 feedback loop to control the cellular effects of mTOR kinase inhibitors in GBM and support the use of DUSP10 expression as a surrogate biomarker to predict responsiveness.
PP242 suppresses bladder cancer cell proliferation and migration through deactivating the mammalian target of rapamycin complex 2/AKT1 signaling pathway.[Pubmed:26548560]
Mol Med Rep. 2016 Jan;13(1):333-8.
While most cancer types are resistant to mammalian target of rapamycin complex 1 (mTORC1) inhibitor rapamycin, recent studies have identified mTORC2 as an important prospective therapeutic target for cancer. The present study assessed the effects of mTORC2 inhibitor PP242 on the proliferation and migration of bladder cancer cells by using Cell Counting Kit8, 5ethynyl2'deoxyuridine incorporation, wound healing and Transwell assays. Furthermore, the phosphorylation status of downstream signaling proteins of mTORC1 and mTORC2 was assessed using western blot analysis. The results demonstrated that PP242 concentrationdependently inhibited the proliferation of bladder cancer cells. Simultaneously, the migration ability of bladder cancer cells was suppressed by PP242. In addition, PP242 markedly restrained the phosphorylation of AKT1 and mTORC2, while the phosphorylation status of S6K1 and mTORC1 was not affected. These results suggested that PP242 exerts potent inhibitory effects on bladder cancer cells by modulating the activity of the mTORC2/AKT1 pathway.
mTOR kinase inhibitor pp242 causes mitophagy terminated by apoptotic cell death in E1A-Ras transformed cells.[Pubmed:26636543]
Oncotarget. 2015 Dec 29;6(42):44905-26.
mTOR is a critical target for controlling cell cycle progression, senescence and cell death in mammalian cancer cells. Here we studied the role of mTOR-dependent autophagy in implementating the antiprolifrative effect of mTORC1-specific inhibitor rapamycin and ATP-competitive mTOR kinase inhibitor PP242. We carried out a comprehensive analysis of PP242- and rapamycin-induced autophagy in ERas tumor cells. Rapamycin exerts cytostatic effect on ERas tumor cells, thus causing a temporary and reversible cell cycle arrest, activation of non-selective autophagy not accompanied by cell death. The rapamycin-treated cells are able to continue proliferation after drug removal. The ATP-competitive mTORC1/mTORC2 kinase inhibitor PP242 is highly cytotoxic by suppressing the function of mTORC1-4EBP1 axis and mTORC1-dependent phosphorylation of mTORC1 target--ULK1-Ser757 (Atg1). In contrast to rapamycin, PP242 activates the selective autophagy targeting mitochondria (mitophagy). The PP242-induced mitophagy is accompanied by accumulation of LC3 and conversion of LC3-I form to LC3-II. However reduced degradation of p62/SQSTM indicates abnormal flux of autophagic process. According to transmission electron microscopy data, short-term PP242-treated ERas cells exhibit numerous heavily damaged mitochondria, which are included in single membrane-bound autophagic/autolysophagic vacuoles (mitophagy). Despite the lack of typical for apoptosis features, ERas-treated cells with induced mitophagy revealed the activation of caspase 3, 9 and nucleosomal DNA fragmentation. Thus, PP242 activates autophagy with suppressed later stages, leading to impaired recycling and accumulation of dysfunctional mitochondria and cell death. Better understanding of how autophagy determines the fate of a cell--survival or cell death, can help to development of new strategy for cancer therapy.
mTOR complex 2 is involved in regulation of Cbl-dependent c-FLIP degradation and sensitivity of TRAIL-induced apoptosis.[Pubmed:23319802]
Cancer Res. 2013 Mar 15;73(6):1946-57.
The mTOR positively regulates cell proliferation and survival through forming 2 complexes with raptor (mTOR complex 1; mTORC1) or rictor (mTOR complex 2; mTORC2). Compared with the mTORC1, relatively little is known about the biologic functions of mTORC2. This study focuses on addressing whether mTORC2 regulates apoptosis, particularly induced by TRAIL (TNFSF10). Using the mTOR kinase inhibitor, PP242, as a research tool, we found that it synergized with TRAIL to augment apoptosis of cancer cells. PP242 reduced the abundance of the short form of c-FLIP (FLIP(S), CFLAR(S)) and survivin (BIRC5). Enforced expression of ectopic FLIP(S), but not survivin, attenuated augmented apoptosis induced by PP242 plus TRAIL. Thus, it is FLIP(S) downregulation that contributes to synergistic induction of apoptosis by PP242 plus TRAIL. PP242 decreased FLIP(S) stability, increased FLIP(S) ubiquitination, and facilitated FLIP(S) degradation. Moreover, knockdown of the E3 ligase Cbl (CBL) abolished PP242-induced FLIP(S) reduction. Thus, PP242 induces Cbl-dependent degradation of FLIP(S), leading to FLIP(S) downregulation. Consistently, knockdown of rictor or mTOR, but not raptor, mimicked PP242 in decreasing FLIP(S) levels and sensitizing cells to TRAIL. Rictor knockdown decreased FLIP(S) stability, whereas enforced expression of rictor stabilized FLIP(S). Moreover, silencing of Cbl abrogated FLIP(S) reduction induced by rictor knockdown. Collectively we conclude that it is mTORC2 inhibition that results in FLIP(S) downregulation and subsequent sensitization of TRAIL-induced apoptosis. Our findings provide the first evidence showing that mTORC2 stabilizes FLIP(S), hence connecting mTORC2 signaling to the regulation of death receptor-mediated apoptosis.
Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor.[Pubmed:20072130]
Nat Med. 2010 Feb;16(2):205-13.
Targeting the mammalian target of rapamycin (mTOR) protein is a promising strategy for cancer therapy. The mTOR kinase functions in two complexes, TORC1 (target of rapamycin complex-1) and TORC2 (target of rapamycin complex-2); however, neither of these complexes is fully inhibited by the allosteric inhibitor rapamycin or its analogs. We compared rapamycin with PP242, an inhibitor of the active site of mTOR in both TORC1 and TORC2 (hereafter referred to as TORC1/2), in models of acute leukemia harboring the Philadelphia chromosome (Ph) translocation. We demonstrate that PP242, but not rapamycin, causes death of mouse and human leukemia cells. In vivo, PP242 delays leukemia onset and augments the effects of the current front-line tyrosine kinase inhibitors more effectively than does rapamycin. Unexpectedly, PP242 has much weaker effects than rapamycin on the proliferation and function of normal lymphocytes. PI-103, a less selective TORC1/2 inhibitor that also targets phosphoinositide 3-kinase (PI3K), is more immunosuppressive than PP242. These findings establish that Ph(+) transformed cells are more sensitive than normal lymphocytes to selective TORC1/2 inhibitors and support the development of such inhibitors for leukemia therapy.
Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases.[Pubmed:18849971]
Nat Chem Biol. 2008 Nov;4(11):691-9.
The clinical success of multitargeted kinase inhibitors has stimulated efforts to identify promiscuous drugs with optimal selectivity profiles. It remains unclear to what extent such drugs can be rationally designed, particularly for combinations of targets that are structurally divergent. Here we report the systematic discovery of molecules that potently inhibit both tyrosine kinases and phosphatidylinositol-3-OH kinases, two protein families that are among the most intensely pursued cancer drug targets. Through iterative chemical synthesis, X-ray crystallography and kinome-level biochemical profiling, we identified compounds that inhibit a spectrum of new target combinations in these two families. Crystal structures revealed that the dual selectivity of these molecules is controlled by a hydrophobic pocket conserved in both enzyme classes and accessible through a rotatable bond in the drug skeleton. We show that one compound, PP121, blocks the proliferation of tumor cells by direct inhibition of oncogenic tyrosine kinases and phosphatidylinositol-3-OH kinases. These molecules demonstrate the feasibility of accessing a chemical space that intersects two families of oncogenes.


