PSB 603Highly selective A2B antagonist CAS# 1092351-10-4 |
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092351-10-4 | SDF | Download SDF |
| PubChem ID | 74944232 | Appearance | Powder |
| Formula | C24H25ClN6O4S | M.Wt | 529.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
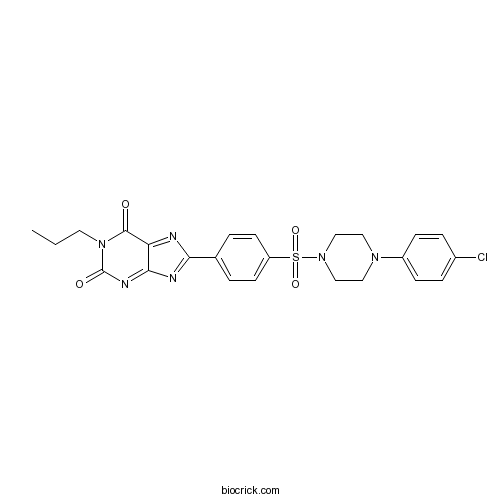
| Chemical Name | 8-[4-[4-(4-chlorophenyl)piperazin-1-yl]sulfonylphenyl]-1-propylpurine-2,6-dione | ||
| SMILES | CCCN1C(=O)C2=NC(=NC2=NC1=O)C3=CC=C(C=C3)S(=O)(=O)N4CCN(CC4)C5=CC=C(C=C5)Cl | ||
| Standard InChIKey | OHVMMGQPJADDAJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H23ClN6O4S/c1-2-11-31-23(32)20-22(28-24(31)33)27-21(26-20)16-3-9-19(10-4-16)36(34,35)30-14-12-29(13-15-30)18-7-5-17(25)6-8-18/h3-10H,2,11-15H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Adenosine A2B receptor antagonist that displays > 17000-fold selectivity over other adenosine receptors (Ki values are 0.553, > 10000, > 10000 and > 10000 nM for A2B, A1, A2A and A3 receptors respectively). |

PSB 603 Dilution Calculator

PSB 603 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8903 mL | 9.4516 mL | 18.9032 mL | 37.8065 mL | 47.2581 mL |
| 5 mM | 0.3781 mL | 1.8903 mL | 3.7806 mL | 7.5613 mL | 9.4516 mL |
| 10 mM | 0.189 mL | 0.9452 mL | 1.8903 mL | 3.7806 mL | 4.7258 mL |
| 50 mM | 0.0378 mL | 0.189 mL | 0.3781 mL | 0.7561 mL | 0.9452 mL |
| 100 mM | 0.0189 mL | 0.0945 mL | 0.189 mL | 0.3781 mL | 0.4726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
The A2b adenosine receptor antagonist PSB-603 promotes oxidative phosphorylation and ROS production in colorectal cancer cells via adenosine receptor-independent mechanism.[Pubmed:27693637]
Cancer Lett. 2016 Dec 1;383(1):135-143.
PURPOSE: Adenosine is a multifaceted regulator of tumor progression. It modulates immune cell activity as well as acting directly on tumor cells. The A2b adenosine receptor (A2b-AR) is thought to be an important mediator of these effects. In this study we sought to analyze the contribution of the A2b-AR to the behavior of colorectal cancer cells. PRINCIPAL RESULTS: The A2b-AR antagonist PSB-603 changed cellular redox state without affecting cellular viability. Quantification of cellular bioenergetics demonstrated that PSB-603 increased basal oxygen consumption rates, indicative of enhanced mitochondrial oxidative phosphorylation. Unexpectedly, pharmacological and genetic approaches to antagonize AR-related signalling of PSB-603 did not abolish the response, suggesting that it was AR-independent. PSB-603 also induced acute increases in reactive oxygen species, and PSB-603 synergized with chemotherapy treatment to increase colorectal cancer cell death, consistent with the known link between cellular metabolism and chemotherapy response. MAJOR CONCLUSIONS: PSB-603 alters cellular metabolism in colorectal cancer cells and increases their sensitivity to chemotherapy. Although requiring more mechanistic insight into its A2b-AR-independent activity, our results show that PSB-603 may have clinical value as an anti-colorectal cancer therapeutic.
1-alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity.[Pubmed:19569717]
J Med Chem. 2009 Jul 9;52(13):3994-4006.
A new series of 1-alkyl-8-(piperazine-1-sulfonyl)phenylxanthines was designed, synthesized, and characterized in radioligand binding and functional assays at A(2B) adenosine receptors. A(2B) antagonists with subnanomolar affinity and high selectivity were discovered. The most potent compounds were 1-ethyl-8-(4-(4-(4-trifluoromethylbenzyl)piperazine-1-sulfonyl)phenyl)xanthine (24, PSB-09120, K(i) (human A(2B)) = 0.157 nM) and 8-(4-(4-(4-chlorobenzyl)piperazine-1-sulfonyl)phenyl)-1-propylxanthine (17, PSB-0788, K(i) (human A(2B)) = 0.393 nM). Moreover, 8-(4-(4-(4-chlorophenyl)piperazine-1-sulfonyl)phenyl)-1-propylxanthine (35, PSB-603) was developed as an A(2B)-specific antagonist exhibiting a K(i) value of 0.553 nM at the human A(2B) receptor and virtually no affinity for the human and rat A(1) and A(2A) and the human A(3) receptors up to a concentration of 10 microM. A tritiated form of the compound was prepared as a new radioligand and characterized in kinetic, saturation, and competition studies. It was shown to be a useful pharmacological tool for the selective labeling of human as well as rodent A(2B) receptors (K(D) human A(2B) 0.403 nM, mouse A(2B) 0.351 nM).


