Poziotinibirreversible pan-HER inhibitor CAS# 1092364-38-9 |
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092364-38-9 | SDF | Download SDF |
| PubChem ID | 25127713 | Appearance | Powder |
| Formula | C23H21Cl2FN4O3 | M.Wt | 491.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HM781-36B; NOV120101 | ||
| Solubility | DMSO : 135 mg/mL (274.76 mM; Need ultrasonic) | ||
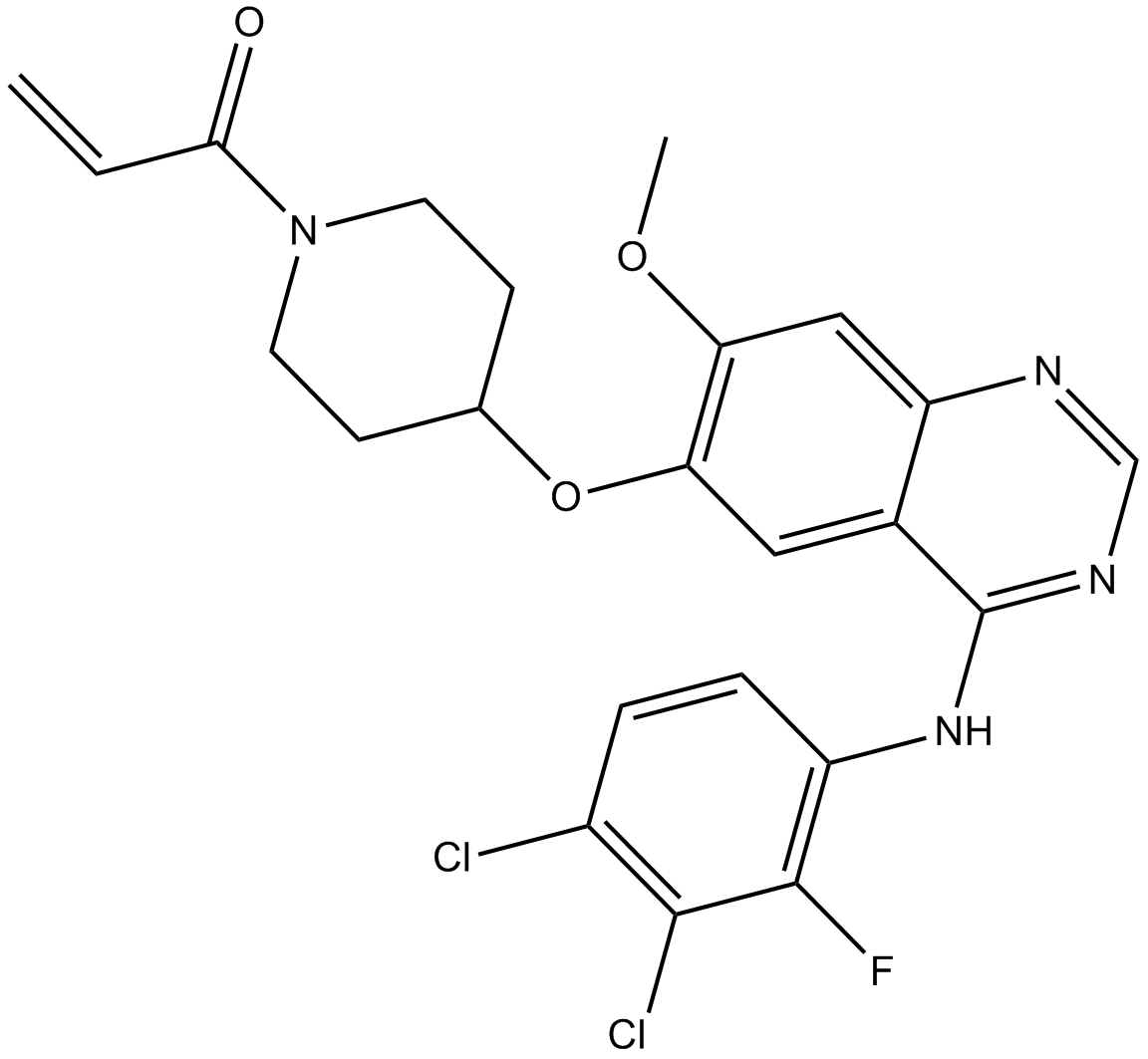
| Chemical Name | 1-[4-[4-(3,4-dichloro-2-fluoroanilino)-7-methoxyquinazolin-6-yl]oxypiperidin-1-yl]prop-2-en-1-one | ||
| SMILES | COC1=C(C=C2C(=C1)N=CN=C2NC3=C(C(=C(C=C3)Cl)Cl)F)OC4CCN(CC4)C(=O)C=C | ||
| Standard InChIKey | LPFWVDIFUFFKJU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Poziotinib(NOV120101; HM781-36B) is an irreversible Pan-HER inhibitor with IC50s of 3/5/23 nM for HER1/HER2/HER4 respectively.
IC50 value: 3/5/23 nM(HER1/HER2/HER4) [1]
Target: pan-HER inhibitor
in vitro: The IC50 levels of HM781-36B for N87 and SNU216 were 0.001 and 0.004 μmol/L, respectively, which was 10–1000 fold lower than the IC50 levels of other HER family TKIs. HM781-36B more potently inhibited the phosphorylation of HER family and downstream proteins, and induced apoptosis and G1 arrest compared to gefitinib or lapatinib [1]. HM781-36B is a potent inhibitor of EGFR in vitro, including the EGFR-acquired resistance mutation (T790M), as well as HER-2 and HER-4, compared with other EGFR tyrosine kinases inhibitors (erlotinib, lapatinib and BIBW2992). HM781-36B treatment of EGFR DelE746_A750-harboring erlotinib-sensitive HCC827 and EGFR L858R/T790M-harboring erlotinib-resistant NCI-H1975 NSCLC cells results in the inhibition of EGFR phosphorylation and the subsequent deactivation of downstream signaling proteins [2]. The addition of HM781-36B induced potent growth inhibition in both DiFi cells with EGFR overexpression and SNU-175 cells (IC50 = 0.003 and 0.005 μM, respectively). Furthermore, HM781-36B induced G1 arrest of the cell cycle and apoptosis, and reduced the levels of HER family and downstream signaling molecules, pERK and pAKT, as well as nonreceptor/cytoplasmic tyrosine kinase, BMX [3].
in vivo: The growth of tumors in mice treated with HM781-36B alone or in combination with 5-FU was significantly inhibited compared with control mice, and tumor volume in mice receiving coadministraion of HM781-36B and 5-FU was smaller than tumor volume in mice receiving HM781-36B only [1]. References: | |||||

Poziotinib Dilution Calculator

Poziotinib Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Poziotinib (HM781-36B) is an irreversible pan-HER inhibitor with IC50 values of 3.2, 5.3 and 23.5 nM for HER1, HER2 and HER4, respectively [1].
Epidermal growth factor receptors (EGFR/HER1, HER2/ErbB2, HER3/ErbB3, HER4/ErbB4) are receptor tyrosine kinases and play important roles in cell proliferation and differentiation. The EGFR family is a major target of anticancer drugs [1].
Poziotinib is an irreversible pan-HER inhibitor. In HER2 amplified SNU216 and N87 gastric cancer cells, HM781-36B inhibited cell growth with IC50 values of 4 and 1 nM, respectively. Also, HM781-36B potently inhibited the phosphorylation of HER family and downstream proteins such as STAT3, AKT and ERK, and induced G1 arrest and apoptosis. HM781-36B dose-dependently increased the amount of cleaved form of effector caspases (caspase-3 and caspase-7) and PARP, reduced antiapoptotic proteins BCL-2 and MCL-1, and induced proapoptotic protein BIM [1]. In HER2-amplified breast cancer cells, HM781-36B inhibited cell growth with IC50 values of 1, 1.2 and 9.5 nM for SK-BR-3, BT474, and MDA-MB-453 cells, respectively [2].
In nude mouse bearing N87 human gastric cancer xenograft model, HM781-36B significantly inhibited tumor growth and exhibited a synergistic effect when administered with chemotherapeutic agents [1].
References:
[1]. Nam HJ, Kim HP, Yoon YK, et al. Antitumor activity of HM781-36B, an irreversible Pan-HER inhibitor, alone or in combination with cytotoxic chemotherapeutic agents in gastric cancer. Cancer Lett, 2011, 302(2): 155-165.
[2]. Kim HJ, Kim HP, Yoon YK, et al. Antitumor activity of HM781-36B, a pan-HER tyrosine kinase inhibitor, in HER2-amplified breast cancer cells. Anticancer Drugs, 2012, 23(3): 288-297.
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
- (3S,4R)-Tofacitinib
Catalog No.:BCC4267
CAS No.:1092578-48-7
- WAY 100635 hydrochloride
Catalog No.:BCC5061
CAS No.:146714-97-8
A Phase II Study of Poziotinib in Patients with Epidermal Growth Factor Receptor (EGFR)-Mutant Lung Adenocarcinoma Who Have Acquired Resistance to EGFR-Tyrosine Kinase Inhibitors.[Pubmed:27188206]
Cancer Res Treat. 2017 Jan;49(1):10-19.
PURPOSE: We examined the efficacy of Poziotinib, a second-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) in patients with lung adenocarcinoma with activating EGFR mutations, who developed acquired resistance (AR) to EGFR-TKIs. MATERIALS AND METHODS: This single-arm phase II study included EGFR-mutant lung adenocarcinoma with AR to erlotinib or gefitinib based on the Jackman criteria. Patients received Poziotinib 16 mg orally once daily in a 28-day cycle. The primary endpoint was progression-free survival (PFS). Prestudy tumor biopsies and blood samples were obtained to determine resistance mechanisms. RESULTS: Thirty-nine patients were treated. Tumor genotyping was determined in 37 patients; 19 EGFR T790M mutations and two PIK3CA mutations were detected in the prestudy tumors, and seven T790M mutations were detected in the plasma assay. Three (8%; 95% confidence interval [CI], 2 to 21) and 17 (44%; 95% CI, 28 to 60) patients had partial response and stable disease, respectively. The median PFS and overall survival were 2.7 months (95% CI, 1.8 to 3.7) and 15.0 months (95% CI, 9.5 to not estimable), respectively. A longer PFS was observed for patients without T790M or PIK3CA mutations in tumor or plasma compared to those with these mutations (5.5 months vs. 1.8 months, p=0.003). The most frequent grade 3 adverse events were rash (59%), mucosal inflammation (26%), and stomatitis (18%). Most patients required one (n=15) or two (n=15) dose reductions. CONCLUSION: Low activity of Poziotinib was detected in patients with EGFR-mutant non-small cell lung cancer who developed AR to gefitinib or erlotinib, potentially because of severe-toxicityimposed dose limitation.
Population pharmacokinetics of HM781-36 (poziotinib), pan-human EGF receptor (HER) inhibitor, and its two metabolites in patients with advanced solid malignancies.[Pubmed:25377158]
Cancer Chemother Pharmacol. 2015 Jan;75(1):97-109.
PURPOSE: To develop a population pharmacokinetic (PK) model for HM781-36 (Poziotinib) and its metabolites in cancer patients. METHODS: Blood samples were collected from three phase I studies in which fifty-two patients received oral HM781-36B tablets (0.5-32 mg) once daily for 2 weeks, and another 20 patients received oral HM781-36B tablets (12, 16, 18, 24 mg) in fasting (12 patients) or fed (eight patients) state once daily for 4 weeks. Nonlinear mixed effect modeling was employed to develop the population pharmacokinetic model. RESULTS: HM781-36 PK was ascribed to a two-compartment model and HM781-36-M1/-M2 PK to one-compartment model. HM781-36 oral absorption was characterized by first-order input (absorption rate constant: 1.45 +/- 0.23 h(-)(1)). The central volume of distribution (185 +/- 12.7 L) was influenced significantly by body weight. The absorption rate constant was influenced by food. The typical HM781-36 apparent clearance was 34.5 L/h (29.4 %CV), with an apparent peripheral volume of distribution of 164 L (53.5 %CV). Other covariates did not significantly further explain the PKs of HM781-36. CONCLUSIONS: The proposed model suggests that HM781-36 PKs are consistent across most solid tumor types, and that the absorption process of HM781-36 is affected by the fed state before dosing. HM781-36 PKs are not complicated by patient factors, other than body weight.


