Deuterated Atazanivir-D3-1CAS# 1092540-56-1 |
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Desmopressin Acetate
Catalog No.:BCC1526
CAS No.:62288-83-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092540-56-1 | SDF | Download SDF |
| PubChem ID | 25144997 | Appearance | Powder |
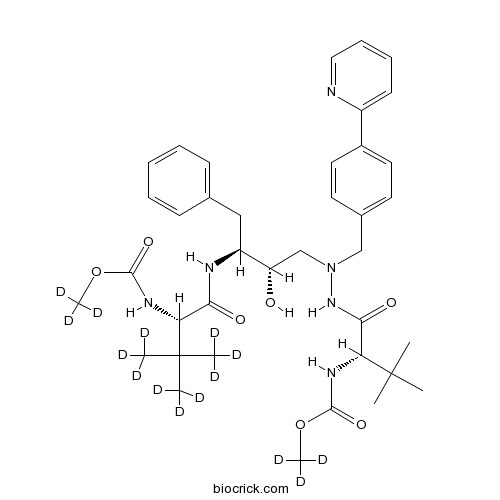
| Formula | C38H37D15N6O7 | M.Wt | 719.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO mg/mL Water mg/mL Ethanol mg/mL | ||
| Chemical Name | trideuteriomethyl N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-4-phenyl-3-[[(2S)-4,4,4-trideuterio-2-(trideuteriomethoxycarbonylamino)-3,3-bis(trideuteriomethyl)butanoyl]amino]butyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate | ||
| SMILES | CC(C)(C)C(C(=O)NC(CC1=CC=CC=C1)C(CN(CC2=CC=C(C=C2)C3=CC=CC=N3)NC(=O)C(C(C)(C)C)NC(=O)OC)O)NC(=O)OC | ||
| Standard InChIKey | AXRYRYVKAWYZBR-ILCNTUBFSA-N | ||
| Standard InChI | InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1/i1D3,2D3,3D3,7D3,8D3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Deuterated Atazanivir-D3-1 Dilution Calculator

Deuterated Atazanivir-D3-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.389 mL | 6.9449 mL | 13.8899 mL | 27.7797 mL | 34.7246 mL |
| 5 mM | 0.2778 mL | 1.389 mL | 2.778 mL | 5.5559 mL | 6.9449 mL |
| 10 mM | 0.1389 mL | 0.6945 mL | 1.389 mL | 2.778 mL | 3.4725 mL |
| 50 mM | 0.0278 mL | 0.1389 mL | 0.2778 mL | 0.5556 mL | 0.6945 mL |
| 100 mM | 0.0139 mL | 0.0694 mL | 0.1389 mL | 0.2778 mL | 0.3472 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Atazanivir-D3-1 is a azapeptide derivative and inhibits HIV protease.
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
- (3S,4R)-Tofacitinib
Catalog No.:BCC4267
CAS No.:1092578-48-7
- WAY 100635 hydrochloride
Catalog No.:BCC5061
CAS No.:146714-97-8
- IT1t dihydrochloride
Catalog No.:BCC6234
CAS No.:1092776-63-0
- PP121
Catalog No.:BCC4980
CAS No.:1092788-83-4
- Ruxolitinib sulfate
Catalog No.:BCC1913
CAS No.:1092939-16-6
- Ruxolitinib phosphate
Catalog No.:BCC1912
CAS No.:1092939-17-7
- 7,2',4'-Trihydroxy-5-methoxy-3-phenylcoumarin
Catalog No.:BCN1626
CAS No.:1092952-62-9
- Angelol M
Catalog No.:BCN8271
CAS No.:1092952-64-1
Deuterated Ethanol as a Probe for Measuring Equilibrium Isotope Effects for Hydroxyl Exchange.[Pubmed:28256831]
J Phys Chem A. 2017 Mar 23;121(11):2288-2292.
Equilibrium deuterium isotope effects for exchange of hydroxyl deuterons and protons among tert-butanol, phenol, ethanethiol, diethylamine, and ethanol were measured by using NMR and also calculated theoretically. Deuterated ethanol could be used as a probe for measuring equilibrium isotope effects (EIE) for hydroxyl exchange; tert-butanol, phenol, ethanethiol, diethylamine, and pyrrole were used as five representive examples. A procedure called the "one-atom isotope effect" was used to save time in the calculations.
Structural study of the novel deuterated calix[4]pyrrole complex d12-meso-tetrakis(4-methoxyphenyl)-meso-tetramethylcalix[4]pyrrole-pyridine N-oxide-acetonitrile (1/1/1).[Pubmed:28257021]
Acta Crystallogr C Struct Chem. 2017 Mar 1;73(Pt 3):254-258.
Calix[4]pyrroles act as powerful receptors for electron-rich neutral guests and anionic guests in organic solvents. For the electron-rich neutral guest pyridine N-oxide, calix[4]pyrrole, with a deep cavity, provides an appropriate environment. The ability of calix[4]pyrrole to host binding guest molecules is the result of hydrogen bonding, pi-pi, C-H...pi and hydrophobic interactions of the cavity. The novel title complex, C52H40D12N4O4.C5H5NO.C2H3N, based on d12-meso-tetrakis(4-methoxyphenyl)-meso-tetramethylcalix[4]pyrrole, has been assembled using an excess of pyridine N-oxide and is the first deuterated complex of calix[4]pyrrole. A single-crystal X-ray study shows that the receptor adopts a cone conformation with the N-oxide fragment encapsulated deep within the cavity. (1)H NMR spectroscopy was used to probe the molecular binding formation in CD3CN. The results are consistent with the single-crystal X-ray study in identifying that the pyridine N-oxide molecule occupies the cavity of the calix[4]pyrrole molecule. UV-vis spectroscopy revealed that the calix[4]pyrrole receptor molecules are able to form 1:1 inclusion complexes in CH3CN.
Synthesis of specific deuterated derivatives of the long chained stratum corneum lipids [EOS] and [EOP] and characterization using neutron scattering.[Pubmed:28370273]
J Labelled Comp Radiopharm. 2017 Jun 15;60(7):316-330.
The synthesis of specific deuterated derivatives of the long chained ceramides [EOS] and [EOP] is described. The structural differences with respect to the natural compounds are founded in the substitution of the 2 double bonds containing linoleic acid by a palmitic acid branched with a methyl group in 10-position. The specific deuteration is introduced both in the branched and in the terminal methyl group, which was realized by common methods of successive deuteration of carboxylic groups in 3 steps. These modified fatty acids resp. the corresponding ceramides [EOS] and [EOP] were prepared for neutron scattering investigations. First results of these investigations were presented in this manuscript showing that the deuterated compounds could be detected in the stratum corneum lipid model membranes. The deuterated ceramides [EOS] and [EOP] are valuable tools to investigate the influence of these long chained ceramide species on the nanostructure of stratum corneum lipid model membranes.
Synthesis of Selectively Substituted or Deuterated Indenes via Sequential Pd and Ru Catalysis.[Pubmed:28332398]
J Org Chem. 2017 Apr 21;82(8):4226-4234.
A strategy for the synthesis of functionalized indenes is presented. The readily available substituted phenols are used as starting materials in the reaction sequence composed of Pd-catalyzed Suzuki coupling and Ru-catalyzed ring-closing metathesis, thus representing a practical method for the controlled construction of functionalized indene derivatives. The methodology has been successfully applied to a broad range of substrates, producing substituted indenes in excellent yields. This approach is also utilized for the synthesis of substituted indenes selectively deuterated in position 3, which are rare in literature.


