Ruxolitinib sulfateJAK inhibitor CAS# 1092939-16-6 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092939-16-6 | SDF | Download SDF |
| PubChem ID | 66577102 | Appearance | Powder |
| Formula | C17H20N6O4S | M.Wt | 404.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | INCB018424 sulfate | ||
| Solubility | DMSO | ||
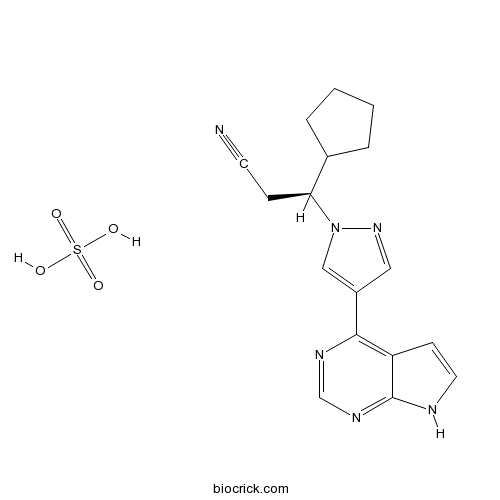
| Chemical Name | (3S)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile;sulfuric acid | ||
| SMILES | C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3.OS(=O)(=O)O | ||
| Standard InChIKey | LGJWVXWQCTZSGC-RSAXXLAASA-N | ||
| Standard InChI | InChI=1S/C17H18N6.H2O4S/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16;1-5(2,3)4/h6,8-12,15H,1-5H2,(H,19,20,21);(H2,1,2,3,4)/t15-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ruxolitinib sulfate is the first potent, selective JAK1/2 inhibitor to enter the clinic with IC50s of 3.3 nM/2.8 nM, and has > 130-fold selectivity for JAK1/2 versus JAK3.In Vitro:Ruxolitinib sulfate is the first potent, selective JAK1/2 inhibitor to enter the clinic with IC50s of 3.3 nM/2.8 nM, and has > 130-fold selectivity for JAK1/2 versus JAK3. Ruxolitinib potently and selectively inhibits JAK2V617F-mediated signaling and proliferation, markedly increases apoptosis in a dose dependent manner, and at 64 nM results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. Ruxolitinib demonstrates remarkable potency against erythroid colony formation with IC50 of 67 nM, and inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 values of 407 nM and 223 nM, respectively[1].In Vivo:Ruxolitinib (180 mg/kg, orally, twice a day) results in survive rate of greater than 90% by day 22 and markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model[1]. In the Ruxolitinib group, the primary end point is reached in 41.9% of patients, as compared with 0.7% in the placebo group in the double-blind trial of myelofibrosis. Ruxolitinib results in maintaining of reduction in spleen volume and improvement of 50% or more in the total symptom score[2]. Ruxolitinib (15 mg twice daily) treatment leads a total of 28% of the patients to have at least a 35% reduction in spleen volume at week 48 in patients with myelofibrosis, as compared with 0% in the group receiving the best available therapy. The mean palpable spleen length has decreased by 56% with Ruxolitinib but has increased by 4% with the best available therapy at week 48. Patients in the ruxolitinib group has an improvement in overall quality-of-life measures and a reduction in symptoms associated with myelofibrosis[3]. References: | |||||

Ruxolitinib sulfate Dilution Calculator

Ruxolitinib sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4726 mL | 12.3628 mL | 24.7255 mL | 49.4511 mL | 61.8139 mL |
| 5 mM | 0.4945 mL | 2.4726 mL | 4.9451 mL | 9.8902 mL | 12.3628 mL |
| 10 mM | 0.2473 mL | 1.2363 mL | 2.4726 mL | 4.9451 mL | 6.1814 mL |
| 50 mM | 0.0495 mL | 0.2473 mL | 0.4945 mL | 0.989 mL | 1.2363 mL |
| 100 mM | 0.0247 mL | 0.1236 mL | 0.2473 mL | 0.4945 mL | 0.6181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
INCB018424 (Ruxolitinib) is an orally bioavailable JAK inhibitor .(IC50 values for JAK1, JAK2 and JAK3 are 3, 5 and 332 nM, respectively).INCB018424 is useful for rheumatoid arthritis.
- PP121
Catalog No.:BCC4980
CAS No.:1092788-83-4
- IT1t dihydrochloride
Catalog No.:BCC6234
CAS No.:1092776-63-0
- WAY 100635 hydrochloride
Catalog No.:BCC5061
CAS No.:146714-97-8
- (3S,4R)-Tofacitinib
Catalog No.:BCC4267
CAS No.:1092578-48-7
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- Ruxolitinib phosphate
Catalog No.:BCC1912
CAS No.:1092939-17-7
- 7,2',4'-Trihydroxy-5-methoxy-3-phenylcoumarin
Catalog No.:BCN1626
CAS No.:1092952-62-9
- Angelol M
Catalog No.:BCN8271
CAS No.:1092952-64-1
- PLpro inhibitor
Catalog No.:BCC5302
CAS No.:1093070-14-4
- B-Raf inhibitor 1
Catalog No.:BCC4182
CAS No.:1093100-40-3
- HNGF6A
Catalog No.:BCC8021
CAS No.:1093111-54-6
- 3-Hydroxy-4,15-dinor-1(5)-xanthen-12,8-olide
Catalog No.:BCN1625
CAS No.:1093207-99-8
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
- Vibralactone B
Catalog No.:BCN6748
CAS No.:1093230-95-5
- SRT2104 (GSK2245840)
Catalog No.:BCC1950
CAS No.:1093403-33-8
- VU 0155041
Catalog No.:BCC7615
CAS No.:1093757-42-6
- gamma-secretase modulator 2
Catalog No.:BCC1584
CAS No.:1093978-89-2
Versican G1 domain enhances adenoviral-mediated transgene expression and can be modulated by inhibitors of the Janus kinase (JAK)/STAT and Src family kinase pathways.[Pubmed:28684419]
J Biol Chem. 2017 Sep 1;292(35):14381-14390.
To examine the biochemical influences that may contribute to the success of gene therapy for ocular disorders, the role of versican, a vitreous component, in adenoviral-mediated transgene expression was examined. Versican is a large chondroitin sulfate-containing, hyaluronic acid-binding proteoglycan present in the extracellular matrix and in ocular vitreous body. Y79 retinoblastoma cells and CD44-negative SK-N-DZ neuroblastoma cells transduced with adenoviral vectors in the presence of versican respond with an activation of transgene expression. Proteolysis of versican generates a hyaluronan-binding G1 domain. The addition of recombinant versican G1 to SK-N-DZ cells results in a similar activation of transgene expression, and treatment with dasatinib, an inhibitor of Src family kinases, also mimics the effects of versican. Enhancement is accompanied by an increase in signal transducer and activator of transcription 5 (STAT5) phosphorylation and is abrogated by treatment with C188-9, a STAT3/5 inhibitor, or with ruxolitinib, a Janus kinase 1/2 (JAK1/2) inhibitor. These data implicate versican G1 in enhancing adenoviral vector transgene expression in a hyaluronic acid-CD44 independent manner that is down-regulated by inhibitors of the JAK/STAT pathway and enhanced by inhibitors of the Src kinase pathway.


