7,2',4'-Trihydroxy-5-methoxy-3-phenylcoumarinCAS# 1092952-62-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092952-62-9 | SDF | Download SDF |
| PubChem ID | 25015742 | Appearance | Yellow powder |
| Formula | C16H12O6 | M.Wt | 300.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
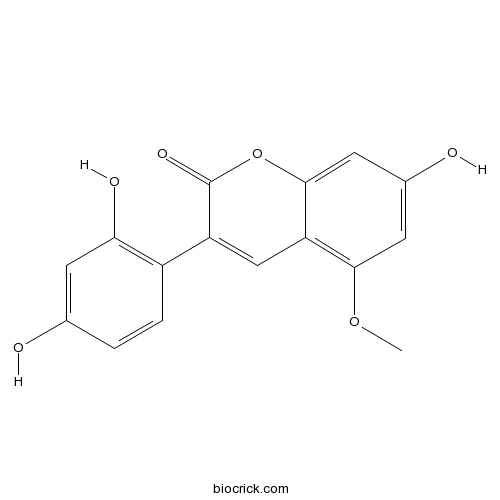
| Chemical Name | 3-(2,4-dihydroxyphenyl)-7-hydroxy-5-methoxychromen-2-one | ||
| SMILES | COC1=C2C=C(C(=O)OC2=CC(=C1)O)C3=C(C=C(C=C3)O)O | ||
| Standard InChIKey | FTOHMMMSWYNATM-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 7,2',4'-Trihydroxy-5-methoxy-3-arylcoumarin shows activity in inhibiting prostate specific antigen (PSA) secreted from androgen dependent prostate cancer cell line, LNCaP cells. |

7,2',4'-Trihydroxy-5-methoxy-3-phenylcoumarin Dilution Calculator

7,2',4'-Trihydroxy-5-methoxy-3-phenylcoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ruxolitinib phosphate
Catalog No.:BCC1912
CAS No.:1092939-17-7
- Ruxolitinib sulfate
Catalog No.:BCC1913
CAS No.:1092939-16-6
- PP121
Catalog No.:BCC4980
CAS No.:1092788-83-4
- IT1t dihydrochloride
Catalog No.:BCC6234
CAS No.:1092776-63-0
- WAY 100635 hydrochloride
Catalog No.:BCC5061
CAS No.:146714-97-8
- (3S,4R)-Tofacitinib
Catalog No.:BCC4267
CAS No.:1092578-48-7
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Angelol M
Catalog No.:BCN8271
CAS No.:1092952-64-1
- PLpro inhibitor
Catalog No.:BCC5302
CAS No.:1093070-14-4
- B-Raf inhibitor 1
Catalog No.:BCC4182
CAS No.:1093100-40-3
- HNGF6A
Catalog No.:BCC8021
CAS No.:1093111-54-6
- 3-Hydroxy-4,15-dinor-1(5)-xanthen-12,8-olide
Catalog No.:BCN1625
CAS No.:1093207-99-8
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
- Vibralactone B
Catalog No.:BCN6748
CAS No.:1093230-95-5
- SRT2104 (GSK2245840)
Catalog No.:BCC1950
CAS No.:1093403-33-8
- VU 0155041
Catalog No.:BCC7615
CAS No.:1093757-42-6
- gamma-secretase modulator 2
Catalog No.:BCC1584
CAS No.:1093978-89-2
- CYM 5442 hydrochloride
Catalog No.:BCC7722
CAS No.:1094042-01-9
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
Urinary 8-Oxo-7,8-Dihydro-2'-Deoxyguanosine in Tunisian Electric Steel Foundry Workers Exposed to Polycyclic Aromatic Hydrocarbons.[Pubmed:28355448]
Ann Work Expo Health. 2017 Apr 1;61(3):333-343.
In this study, urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG), as biomarker of oxidative DNA damage, was evaluated in Tunisian electric steel foundry workers and was associated with polycyclic aromatic hydrocarbon (PAH) exposure. Ninety-three healthy male workers were enrolled in the study; 8-oxodG was assessed by liquid chromatography-triple quadrupole mass spectrometry. Exposure to PAHs was evaluated by measuring 16 urinary PAHs (U-PAHs) and 8 monohydroxylated metabolites (OHPAHs). The median 8-oxodG level for all subjects was 3.20 microg l-1 (1.85 microg g-1 creatinine). No correlation between 8-oxodG and 1-hydroxypyrene or any other OHPAH was found. Significant linear correlations between 8-oxodG and some U-PAHs were found, particularly urinary acenaphthylene (r = 0.249), phenanthrene (r = 0.327), anthracene (r = 0.357), fluoranthene (r = 0.248), and pyrene (r = 0.244). Multiple regression analyses confirmed that urinary phenanthrene, anthracene, and naphthalene (the latter with a non-linear relationship) were predictors of 8-oxodG; job title, but not smoking, was a determinant of 8-oxodG; the variance explained by these models was up to 20%. The oxidative DNA damage assessed by urinary 8-oxodG was moderate and in the range of values reported in other occupational fields or in the general population. The results of this study indicate that the investigated biomarkers of PAH exposure were only minor contributors to urinary 8-oxodG.
Erratum: Gu, S. et al. Error-Free Bypass of 7,8-dihydro-8-oxo-2'-deoxyguanosine by DNA Polymerase of Pseudomonas aeruginosa Phage PaP1. Genes 2017, 8, 18.[Pubmed:28273830]
Genes (Basel). 2017 Mar 3;8(3). pii: genes8030091.
n/a.
The anti/syn conformation of 8-oxo-7,8-dihydro-2'-deoxyguanosine is modulated by Bacillus subtilis PolX active site residues His255 and Asn263. Efficient processing of damaged 3'-ends.[Pubmed:28254425]
DNA Repair (Amst). 2017 Apr;52:59-69.
8-oxo-7,8-dihydro-2'-deoxyguanosine (8oxodG) is a major lesion resulting from oxidative stress and found in both DNA and dNTP pools. Such a lesion is usually removed from DNA by the Base Excision Repair (BER), a universally conserved DNA repair pathway. 8oxodG usually adopts the favored and promutagenic syn-conformation at the active site of DNA polymerases, allowing the base to hydrogen bonding with adenine during DNA synthesis. Here, we study the structural determinants that affect the glycosidic torsion-angle of 8oxodGTP at the catalytic active site of the family X DNA polymerase from Bacillus subtilis (PolXBs). We show that, unlike most DNA polymerases, PolXBs exhibits a similar efficiency to stabilize the anti and syn conformation of 8oxodGTP at the catalytic site. Kinetic analyses indicate that at least two conserved residues of the nucleotide binding pocket play opposite roles in the anti/syn conformation selectivity, Asn263 and His255 that favor incorporation of 8oxodGMP opposite dA and dC, respectively. In addition, the presence in PolXBs of Mn(2+)-dependent 3'-phosphatase and 3'-phosphodiesterase activities is also shown. Those activities rely on the catalytic center of the C-terminal Polymerase and Histidinol Phosphatase (PHP) domain of PolXBs and, together with its 3'-5' exonuclease activity allows the enzyme to resume gap-filling after processing of damaged 3' termini.
Preparation and Practical Applications of 2',7'-Dichlorodihydrofluorescein in Redox Assays.[Pubmed:28224799]
Anal Chem. 2017 Apr 4;89(7):3853-3857.
Oxidative stress, a state in which intra- or extracellular oxidant production outweighs the antioxidative capacity, lies at the basis of many diseases. DCFH2-DA (2',7'-dichlorodihydrofluorescein diacetate) is the most widely used fluorogenic probe for the detection of general oxidative stress. However, the use of DCFH2-DA, as many other fluorogenic redox probes, is mainly confined to the detection of intracellular oxidative stress in vitro. To expand the applicability of the probe, an alkaline hydrolysis and solvent extraction procedure was developed to generate high-purity DCFH2 (2',7'-dichlorodihydrofluorescein) from DCFH2-DA using basic laboratory equipment. Next, the utility of DCFH2 was exemplified in a variety of cell-free and in vitro redox assay systems, including oxidant production by transition metals, photodynamic therapy, activated macrophages, and platelets, as well as the antioxidative capacity of different antioxidants. In cells, the concomitant use of DCFH2-DA and DCFH2 enabled the measurement and compartmentalized analysis of intra- and extracellularly produced oxidants, respectively, using a single read-out parameter. Furthermore, hepatocyte-targeted liposomes were developed to deliver the carboxylated derivative, 5(6)-carboxy-DCFH2, to hepatocytes in vivo. Liposome-delivered 5(6)-carboxy-DCFH2 enabled real-time visualization and measurement of hepatocellular oxidant production during liver ischemia-reperfusion. The liposomal 5(6)-carboxy-DCFH2 can be targeted to other tissues where oxidative stress is important, including cancer.


