TachiosideCAS# 109194-60-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109194-60-7 | SDF | Download SDF |
| PubChem ID | 11962143 | Appearance | Powder |
| Formula | C13H18O8 | M.Wt | 302.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
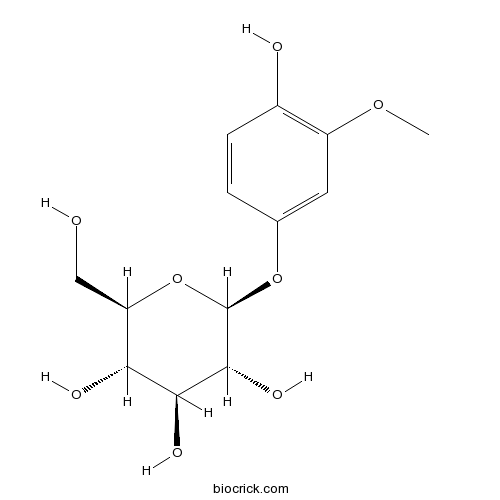
| Chemical Name | (2S,3R,4S,5S,6R)-2-(4-hydroxy-3-methoxyphenoxy)-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=C(C=CC(=C1)OC2C(C(C(C(O2)CO)O)O)O)O | ||
| Standard InChIKey | KWVHACHAQJFTLZ-UJPOAAIJSA-N | ||
| Standard InChI | InChI=1S/C13H18O8/c1-19-8-4-6(2-3-7(8)15)20-13-12(18)11(17)10(16)9(5-14)21-13/h2-4,9-18H,5H2,1H3/t9-,10-,11+,12-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tachioside has antioxidant and α-glucosidase inhibitory activities. 2. Tachioside decreases lipid content in 3T3-L1 adipocytes by inhibiting lipogenesis, shows antiobesity activities. 3. Tachioside can inhibit nitric oxide production in lipopolysaccharides-stimulated RAW 264.7 cells with IC50 value of 12.14 μM. |
| Targets | NO |

Tachioside Dilution Calculator

Tachioside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
Phenolic glycosides from Glycosmis pentaphylla.[Pubmed:25367455]
J Asian Nat Prod Res. 2014 Dec;16(12):1119-25.
Three new phenolic glycosides, named as glycopentosides A-C (1-3), along with nine known compounds were isolated from the n-BuOH extract of stems of Glycosmis pentaphylla. Their structures were determined by using spectroscopic and chemical methods. Bioassay showed that compound 10 (Tachioside) could inhibit nitric oxide production in lipopolysaccharides-stimulated RAW 264.7 cells with IC50 value of 12.14 muM.
A new phenylpropanoid and an alkylglycoside from Piper retrofractum leaves with their antioxidant and alpha-glucosidase inhibitory activity.[Pubmed:25127165]
Bioorg Med Chem Lett. 2014 Sep 1;24(17):4120-4.
Two new compounds, piperoside (1) and isoheptanol 2(S)-O-beta-D-xylopyranosyl (1-->6)-O-beta-D-glucopyranoside (11), along with 10 known compounds 3,4-dihydroxyallylbenzene (2), 1,2-di-O-beta-D-glucopyranosyl-4-allylbenzene (3), Tachioside (4), benzyl-O-beta-D-glucopyranoside (5), icariside F2 (6), dihydrovomifoliol-3'-O-beta-D-glucopyranoside (7), isopropyl O-beta-D-glucopyranoside (8), isopropyl primeveroside (9), n-butyl O-beta-D-glucopyranoside (10), isoheptanol 2(S)-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside (12), were isolated from the leaves of Piper retrofractum. Their structures were determined from 1D-NMR, 2D-NMR, and HR-ESI-MS spectral, a modified Mosher's method, and comparisons with previous reports. All of the isolated compounds showed modest alpha-glucosidase inhibitory (4.60+/-1.74% to 11.97+/-3.30%) and antioxidant activities under the tested conditions.


