N-Valeric acidCAS# 109-52-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109-52-4 | SDF | Download SDF |
| PubChem ID | 7991 | Appearance | Powder |
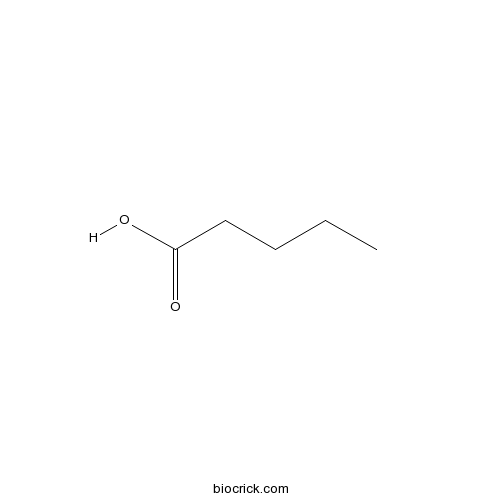
| Formula | C5H10O2 | M.Wt | 102 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 250 mg/mL (2447.86 mM) H2O : 5 mg/mL (48.96 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | pentanoic acid | ||
| SMILES | CCCCC(=O)O | ||
| Standard InChIKey | NQPDZGIKBAWPEJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H10O2/c1-2-3-4-5(6)7/h2-4H2,1H3,(H,6,7) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Experimental study of the hydrothermal reactivity of organic acids and acid anions: II. Acetic acid, acetate, and valeric acid[Reference: WebLink]Geochimica Et Cosmochimica Acta, 2003, 67(19):3645-3664.
Organic acids and acid anions occur in substantial concentrations in many aqueous geologic fluids and are thought to take part in a variety of geochemical processes ranging from the transport of metals in ore-forming fluids to the formation of natural gas to serving as a metabolic energy source for microbes in subsurface habitats.
The widespread occurrence of organic acids and their potential role in diverse geologic processes has led to numerous experimental studies of their thermal stability, yet there remain substantial gaps in our knowledge of the factors that control the rates and reaction pathways for the decomposition of these compounds under geologic conditions.
|

N-Valeric acid Dilution Calculator

N-Valeric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.8039 mL | 49.0196 mL | 98.0392 mL | 196.0784 mL | 245.098 mL |
| 5 mM | 1.9608 mL | 9.8039 mL | 19.6078 mL | 39.2157 mL | 49.0196 mL |
| 10 mM | 0.9804 mL | 4.902 mL | 9.8039 mL | 19.6078 mL | 24.5098 mL |
| 50 mM | 0.1961 mL | 0.9804 mL | 1.9608 mL | 3.9216 mL | 4.902 mL |
| 100 mM | 0.098 mL | 0.4902 mL | 0.9804 mL | 1.9608 mL | 2.451 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
Sorptive process and breakthrough behavior of odorous volatile compounds on inert surfaces.[Pubmed:30177843]
Sci Rep. 2018 Sep 3;8(1):13118.
The use of glass impinger is an important device for sampling and handling when measuring volatile organic compounds (SVOCs). Thus, it is important to check for possible analyte losses to the inner glass surface when carrying out sample analysis with the aid of impinger system. In this research, we evaluated the sorptive loss patterns of vapor-phase semi-volatile organic compounds [SVOCs (n = 10): acetic acid (ACA), propionic acid (PPA), i-butyric acid (IBA), n-butyric acid (BTA), i-valeric acid (IVA), N-Valeric acid (VLA), phenol (PhAl), p-cresol (p-C), indole (ID), and skatole (SK)] on inert surfaces of an impinger in reference to sampling bags. The gaseous standard of these SVOCs (48-406 ppb) in polyester aluminum (PEA) bags was passed through an empty impinger in 1 L steps. The exiting SVOCs were collected on three-bed sorbent tubes for subsequent analysis by thermal desorption-gas chromatography-mass spectroscopy (TD-GC-MS). Impinger wall sorption capacities ranged from 2.0 to 21.0 ng cm(-2). The 10% breakthrough adsorption capacities on the impinger wall for acids, phenols, and indoles ranged from 1.21 +/- 0.15 to 5.39 +/- 0.79, 0.92 +/- 0.12 to 13.4 +/- 2.25, and 4.47 +/- 0.42 to 5.23 +/- 0.35 ng cm(-2), respectively. The observed sorptive patterns suggest that the sorptive losses of the volatile fatty acids, phenols, and indoles can occur very effectively at low ppb levels onto a glass surface.
Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota.[Pubmed:29352879]
Carbohydr Polym. 2018 Mar 1;183:230-239.
The aim of the present study was to investigate structural characteristic and in vitro fermentation of a novel polysaccharide named ST-P2 from Sargassum thunbergii by human fecal inoculums, and its impact on human colonic microbiota. The results showed that ST-P2 was homogeneous with molecular weight of 48,788Da, and consisted of arabinose, galactose, glucose, xylose, and mannose. The main linkage types were identified as (1-->5)-alpha-L-Araf, (1-->3)-alpha-L-Manp, (1-->3,6)-beta-D-Galp, (1-->6)-alpha-D-Glcp, and (1-->3)-beta-D-Xylp, respectively. After 48h fermentation, 67.83+/-1.15% of total carbohydrate was utilized by colonic microbiota. The pH value in the fecal culture significantly decreased from 6.09+/-0.11 to 4.70+/-0.04. The concentrations of total short chain fatty acids, acetic, propionic, n-butyric and N-Valeric acids significantly increased compared to the blank. ST-P2 could remarkably modulate the composition and abundance of beneficial microbiota. These results suggest that ST-P2 could potentially be a functional food aimed at promoting the gut health.
Examination of the molecular control of ruminal epithelial function in response to dietary restriction and subsequent compensatory growth in cattle.[Pubmed:27651894]
J Anim Sci Biotechnol. 2016 Sep 15;7:53.
BACKGROUND: The objective of this study was to investigate the effect of dietary restriction and subsequent compensatory growth on the relative expression of genes involved in volatile fatty acid transport, metabolism and cell proliferation in ruminal epithelial tissue of beef cattle. Sixty Holstein Friesian bulls (mean liveweight 370 +/- 35 kg; mean age 479 +/- 15 d) were assigned to one of two groups: (i) restricted feed allowance (RES; n = 30) for 125 d (Period 1) followed by ad libitum access to feed for 55 d (Period 2) or (ii) ad libitum access to feed throughout (ADLIB; n = 30). Target growth rate for RES was 0.6 kg/d during Period 1. At the end of each dietary period, 15 animals from each treatment group were slaughtered and ruminal epithelial tissue and liquid digesta harvested from the ventral sac of the rumen. Real-time qPCR was used to quantify mRNA transcripts of 26 genes associated with ruminal epithelial function. Volatile fatty acid analysis of rumen fluid from individual animals was conducted using gas chromatography. RESULTS: Diet x period interactions were evident for genes involved in ketogenesis (BDH2, P = 0.017), pyruvate metabolism (LDHa, P = 0.048; PDHA1, P = 0.015) and cellular transport and structure (DSG1, P = 0.019; CACT, P = 0.027). Ruminal concentrations of propionic acid (P = 0.018) and N-Valeric acid (P = 0.029) were lower in RES animals, compared with ADLIB, throughout the experiment. There was also a strong tendency (P = 0.064) toward a diet x period interaction for n-butyric with higher concentrations in RES animals, compared with ADLIB, during Period 1. CONCLUSIONS: These data suggest that following nutrient restriction, the structural integrity of the rumen wall is compromised and there is upregulation of genes involved in the production of ketone bodies and breakdown of pyruvate for cellular energy. These results provide an insight into the potential molecular mechanisms regulating ruminal epithelial absorptive metabolism and growth following nutrient restriction and subsequent compensatory growth.
Extrusion of barley and oat influence the fecal microbiota and SCFA profile of growing pigs.[Pubmed:26758043]
Food Funct. 2016 Feb;7(2):1024-32.
The effect of extrusion of barley and oat on the fecal microbiota and the formation of SCFA was evaluated using growing pigs as model system. The pigs were fed a diet containing either whole grain barley (BU), oat groat (OU), or their respective extruded samples (BE and OE). 454 pyrosequencing showed that the fecal microbiota of growing pigs was affected by both extrusion and grain type. Extruded grain resulted in lower bacterial diversity and enrichment in operational taxonomic units (OTUs) affiliated with members of the Streptococcus, Blautia and Bulleidia genera, while untreated grain showed enrichment in OTUs affiliated with members of the Bifidobacterium and Lactobacillus genera, and the butyrate-producing bacteria Butyricicoccus, Roseburia, Coprococcus and Pseudobutyrivibrio. Untreated grain resulted in a significant increase of n-butyric, i-valeric and N-Valeric acid, which correlated with an increase of Bifidobacterium and Lactobacillus. This is the first study showing that cereal extrusion affects the microbiota composition and diversity towards a state generally thought to be less beneficial for health, as well as less amounts of beneficial butyric acid.
A simple method for the accurate determination of the Henry's law constant for highly sorptive, semivolatile organic compounds.[Pubmed:26577086]
Anal Bioanal Chem. 2016 Jan;408(3):775-84.
A novel technique is developed to determine the Henry's law constants (HLCs) of seven volatile fatty acids (VFAs) with significantly high solubility using a combined application of thermal desorber/gas chromatography/mass spectrometry (TD/GC/MS). In light of the strong sorptive properties of these semi-volatile organic compounds (SVOCs), their HLCs were determined by properly evaluating the fraction lost on the surface of the materials used to induce equilibrium (vial, gas-tight syringe, and sorption tube). To this end, a total of nine repeated experiments were conducted in a closed (static) system at three different gas/liquid volume ratios. The best estimates for HLCs (M/atm) were thus 7,200 (propionic acid), 4,700 (i-butyric acid), 4,400 (n-butyric acid), 2,700 (i-valeric acid), 2,400 (N-Valeric acid), 1,000 (hexanoic acid), and 1,500 (heptanoic acid). The differences in the HLC values between this study and previous studies, if assessed in terms of the percent difference, ranged from 9.2% (N-Valeric acid) to 55.7% (i-valeric acid). We overcame the main cause of errors encountered in previous studies by performing the proper correction of the sorptive losses of the SVOCs that inevitably took place, particularly on the walls of the equilibration systems (mainly the headspace vial and/or the glass tight syringe).
A novel method to quantify the emission and conversion of VOCs in the smoking of electronic cigarettes.[Pubmed:26553711]
Sci Rep. 2015 Nov 10;5:16383.
An analytical technique was developed for the quantitation of volatile organic compounds (VOC) in three different forms of electronic cigarette (EC): solution, vapor, and aerosol. Through the application of the mass change tracking (MCT) approach, the consumed amount of the solution was measured to track the conversion of targets between the different phases. The concentration of aerosol plus vapor (A&V) decreased exponentially (559 to 129 g m(-3)) with increasing puff velocity (0.05 to 1 L min(-1)). A strong correlation existed between sampling volume and consumed solution mass (R(2) = 0.9972 +/- 0.0021 (n = 4)). In the EC solution, acetic acid was considerably high (25.8 mug mL(-1)), along with trace quantities of some VOCs (methyl ethyl ketone, toluene, propionic acid, and i-butyric acid: 0.24 +/- 0.15 mug mL(-1) (n = 4)). In the aerosol samples, many VOCs (n-butyraldehyde, n-butyl acetate, benzene, xylene, styrene, N-Valeric acid, and n-hexanoic acid) were newly produced (138 +/- 250 mug m(-3)). In general, the solution-to-aerosol (S/A) conversion was significant: e.g., 1,540% for i-butyric acid. The emission rates of all targets computed based on their mass in aerosol/ consumed solution (ng mL(-1)) were from 30.1 (p-xylene) to 398 (methyl ethyl ketone), while those of carboxyls were much higher from 166 (acetic acid) to 5,850 (i-butyric acid).
Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis.[Pubmed:25898090]
Bioresour Technol. 2015 Aug;189:279-284.
Hydrogen production during dark fermentation is inhibited by the co-production of volatile fatty acids (VFAs) such as acetic and n-butyric acid. In this study, the effectiveness of conventional electrodialysis (CED) in reducing VFA concentrations in model solutions and hydrogen fermentation broths is evaluated. This is the first time CED has been reported to remove VFAs from hydrogen fermentation broths. During 60 min of operation CED removed up to 99% of VFAs from model solutions, sucrose-fed and grass-fed hydrogen fermentation broths, containing up to 1200 mg l(-1) each of acetic acid, propionic acid, i-butyric acid, n-butyric acid, i-valeric acid, and N-Valeric acid. CED's ability to remove VFAs from hydrogen fermentation broths suggests that this technology is capable of improving hydrogen yields from dark fermentation.
An assessment of the liquid-gas partitioning behavior of major wastewater odorants using two comparative experimental approaches: liquid sample-based vaporization vs. impinger-based dynamic headspace extraction into sorbent tubes.[Pubmed:24271272]
Anal Bioanal Chem. 2014 Jan;406(2):643-55.
The gas-liquid partitioning behavior of major odorants (acetic acid, propionic acid, isobutyric acid, n-butyric acid, i-valeric acid, N-Valeric acid, hexanoic acid, phenol, p-cresol, indole, skatole, and toluene (as a reference)) commonly found in microbially digested wastewaters was investigated by two experimental approaches. Firstly, a simple vaporization method was applied to measure the target odorants dissolved in liquid samples with the aid of sorbent tube/thermal desorption/gas chromatography/mass spectrometry. As an alternative method, an impinger-based dynamic headspace sampling method was also explored to measure the partitioning of target odorants between the gas and liquid phases with the same detection system. The relative extraction efficiency (in percent) of the odorants by dynamic headspace sampling was estimated against the calibration results derived by the vaporization method. Finally, the concentrations of the major odorants in real digested wastewater samples were also analyzed using both analytical approaches. Through a parallel application of the two experimental methods, we intended to develop an experimental approach to be able to assess the liquid-to-gas phase partitioning behavior of major odorants in a complex wastewater system. The relative sensitivity of the two methods expressed in terms of response factor ratios (RFvap/RFimp) of liquid standard calibration between vaporization and impinger-based calibrations varied widely from 981 (skatole) to 6,022 (acetic acid). Comparison of this relative sensitivity thus highlights the rather low extraction efficiency of the highly soluble and more acidic odorants from wastewater samples in dynamic headspace sampling.
Hydrolysis and acidification of dewatered sludge under mesophilic, thermophilic and extreme thermophilic conditions: effect of pH.[Pubmed:24077155]
Bioresour Technol. 2013 Nov;148:461-6.
This study investigated the effect of pH (uncontrolled, 8.0, 10.0 and 12.0) and temperature (mesophilic, thermophilic and extreme thermophilic) on hydrolysis and acidification of dewatered sludge in 7-day batch fermentation experiment. Solublization of COD, protein and carbohydrates as well as concentration and composition of VFAs were investigated. Sludge hydrolysis was enhanced with higher pH and temperature. The maximum SCOD, soluble protein and carbohydrates was observed at pH 12.0 at extreme thermophilic condition. The maximum VFAs yield was obtained at thermophilic and was 2.15 times that at mesophilic condition, but it took more time to reach the maximum. The VFAs consisted of acetic, propionic, iso-butyric, n-butyric, iso-valeric, and N-Valeric acids, and acetic acid was the prevalent product in most cases except for uncontrolled pH and pH 8.0 at mesophilic condition. The methane production was as follows: pH 8.0>pH 10.0>uncontrolled (0.015)>pH 12.0; mesophilic>thermophilic>extreme thermophilic.
Parallel analysis of volatile fatty acids, indole, skatole, phenol, and trimethylamine from waste-related source environments.[Pubmed:24070624]
J Chromatogr A. 2013 Nov 1;1314:241-8.
An experimental technique based on sorbent tube-thermal desorption-gas chromatography (ST-TD-GC) was investigated for the simultaneous determination of a cluster of eight volatile odorants (propionic acid, n-butyric acid, i-valeric acid, N-Valeric acid, trimethylamine, phenol, indole, and skatole) and a reference compound (benzene). Calibration was made by direct injection of a liquid working standard (L-WS) into a quartz tube packed with three bed sorbent (Tenax TA, Carbopack B, and Carbopack X). To assess the relative performance between different detector systems, a comparative analysis was made using both mass spectrometry (MS) and a flame ionization detector (FID) with the aid of a TD system. In the TD-GC-MS analysis, calibration results were evaluated in two different modes, namely total ion chromatogram (TIC) and extracted ion chromatogram (EIC). In both FID and MS, the elution order of investigated odorants complied with the retention time index (RTI) values for the polar column with a coefficient of determination (R(2)) at or above 0.99. As a means to validate our detection approach, environmental samples from a bathroom and manhole (vacuum samples) as well as cat stool and wastewater (headspace samples) were also collected. The ST-TD method tested for the concurrent analysis of diverse odorants allowed us to measure a list of offensive odorants from those samples.
Extent of sample loss on the sampling device and the resulting experimental biases when collecting volatile fatty acids (VFAs) in air using sorbent tubes.[Pubmed:23869450]
Anal Chem. 2013 Aug 20;85(16):7818-25.
Not all volatile organic compounds (VOCs) are suitable for sampling from air onto sorbent tubes (ST) with subsequent analysis by thermal desorption (TD) with gas chromatography (GC). Some compounds (such as C2 hydrocarbons) are too volatile for quantitative retention by sorbents at ambient temperature, while others are too reactive - either for storage stability on the tubes (post-sampling) or for thermal desorption/GC analysis. Volatile fatty acids (VFAs) are one of the compound groups that present a challenge to sorbent tube sampling. In this study, we evaluated sample losses on the inner wall surface of the sorbent tube sampler. The sorptive losses of five VFA (acetic, propionic, n-butyric, i-valeric, and N-Valeric acid) were tested using two types of tubes (stainless steel and quartz), each packed with three sorbent beds arranged in order of sorbent strength from the sampling end of the tube (Tenax TA, Carbopack B, and Carbopack X). It showed significantly higher losses of VFAs in both liquid phase and vapor phase when using stainless steel tube samplers. These losses were also seen if vapor-phase fatty acids were passed through empty stainless steel tubing and increased dramatically with increasing molecular weight, e.g., losses of 33.6% (acetic acid) to 97.5% (N-Valeric acid). Similar losses of VFAs were also observed from headspace sampling of cheese products. Considering that stainless steel sampling tubes are still used extensively by many researchers, their replacement with quartz tubes is recommended to reduce systematic biases in collecting VFA samples or in their calibration.
The repelling effect of plant secondary metabolites on water voles, Arvicola amphibius.[Pubmed:23225271]
Pest Manag Sci. 2013 Mar;69(3):437-43.
BACKGROUND: Water voles (Arvicola amphibius Linnaeus 1758) are abundant in most parts of Germany and other European countries. They are known to cause serious damage in fruit and horticulture as well as in agriculture. Currently available repellents, scaring devices and household remedies are mostly inefficient. Tests were conducted to establish whether water voles can be repelled using plant secondary metabolites. These compounds are produced by many plant species as part of their defence against herbivores and pathogens. RESULTS: In this study, 12 volatile substances were tested in T-maze trials. The voles could choose between a test box including a test substance and a control box without odour. The extracts were considered to be repellent if the test box was avoided. Five potential repellents were identified: the essential oils of black pepper oil, Chinese geranium oil and onion, as well as the pure substances methyl nonyl ketone and N-Valeric acid. Application of a combination of black pepper oil, Chinese geranium oil and methyl nonyl ketone did not increase efficacy. CONCLUSION: The identification of an effective water vole repellent could help to reduce damage to crops. It may also minimise the use of kill traps and of rodenticides, which will be of benefit for non-target organisms.


