GSK1904529ASelective IGF-1R/IR inhibitor CAS# 1089283-49-7 |
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
- Linsitinib
Catalog No.:BCC3697
CAS No.:867160-71-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1089283-49-7 | SDF | Download SDF |
| PubChem ID | 25124816 | Appearance | Powder |
| Formula | C44H47F2N9O5S | M.Wt | 851.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK-1904529A; GSK 1904529A | ||
| Solubility | DMSO : 50 mg/mL (58.69 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
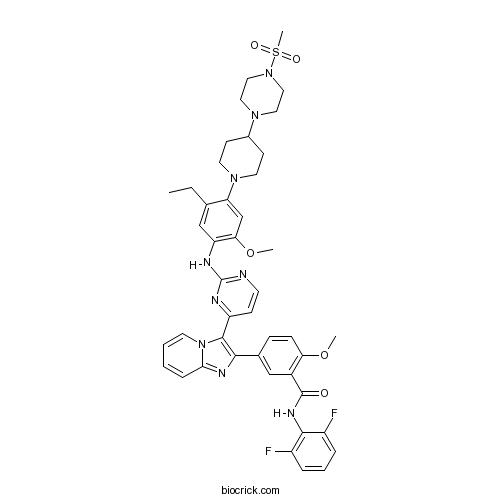
| Chemical Name | N-(2,6-difluorophenyl)-5-[3-[2-[5-ethyl-2-methoxy-4-[4-(4-methylsulfonylpiperazin-1-yl)piperidin-1-yl]anilino]pyrimidin-4-yl]imidazo[1,2-a]pyridin-2-yl]-2-methoxybenzamide | ||
| SMILES | CCC1=CC(=C(C=C1N2CCC(CC2)N3CCN(CC3)S(=O)(=O)C)OC)NC4=NC=CC(=N4)C5=C(N=C6N5C=CC=C6)C7=CC(=C(C=C7)OC)C(=O)NC8=C(C=CC=C8F)F | ||
| Standard InChIKey | MOSKATHMXWSZTQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GSK1904529A is a selective inhibitor of IGF1R with IC50 of 27 nM. | |||||
| Targets | IGF1R | |||||
| IC50 | 27 nM | |||||

GSK1904529A Dilution Calculator

GSK1904529A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1738 mL | 5.8688 mL | 11.7376 mL | 23.4753 mL | 29.3441 mL |
| 5 mM | 0.2348 mL | 1.1738 mL | 2.3475 mL | 4.6951 mL | 5.8688 mL |
| 10 mM | 0.1174 mL | 0.5869 mL | 1.1738 mL | 2.3475 mL | 2.9344 mL |
| 50 mM | 0.0235 mL | 0.1174 mL | 0.2348 mL | 0.4695 mL | 0.5869 mL |
| 100 mM | 0.0117 mL | 0.0587 mL | 0.1174 mL | 0.2348 mL | 0.2934 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK1904529A is a small-molecule inhibitor of the insulin-like growth factor-I receptor (IGF-IR) with IC50 value of 27 nM 1.
GSK1904529A is a reversible and ATP-competitive inhibitor with Ki value of 1.6 nM. In NIH-3T3/LISN cells, GSK1904529A potently inhibited phosphorylation of IGF-IR with IC50 value of 22 nM. It also demonstrated to be a selective inhibitor since it showed poor inhibitory activity against 45 other serine/threonine and tyrosine kinases. When treated with whole-cell extracts, GSK1904529A significantly inhibited the ligand-induced phosphorylation of IGF-IR and decreased phosphorylation of downstream signaling including AKT, IRS-1 and ERK at concentrations > 0.01μM. GSK1904529A suppressed cell proliferation in a variety of tumor cells. The IC50 values for NCI-H929, TC-71, SK-N-MC, COLO 205, MCF7 and PREC are 81, 35, 43, 124, 137 and 68 nM, respectively. In COLO 205, MCF-7, and NCI-H929 cells, GSK1904529A treatment resulted in cell accumulation in G1 and decrease in S and G2-M phases. Moreover, in NIH-3T3/LISN xenograft model, once daily administration of GSK1904529A at 30 mg/kg inhibited 56% of tumor growth 1.
References:
1. Sabbatini P, Rowand J L, Groy A, et al. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase. Clinical Cancer Research, 2009, 15(9): 3058-3067.
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase.[Pubmed:19383820]
Clin Cancer Res. 2009 May 1;15(9):3058-67.
PURPOSE: Dysregulation of the insulin-like growth factor-I receptor (IGF-IR) signaling pathway has been implicated in the development of many types of tumors, including prostate, colon, breast, pancreatic, ovarian, and sarcomas. Agents that inhibit IGF-IR activity may be useful in treatment of patients with various cancers. EXPERIMENTAL DESIGN: Kinase assays were used to identify a selective small-molecule inhibitor of IGF-IR activity. The effects of this compound on IGF-IR signaling, cell proliferation, and the cell cycle were determined using a panel of cell lines. Antitumor activity was evaluated in human tumor xenografts growing in athymic mice. Inhibition of IGF-IR and the closely related insulin receptor (IR) was measured in vivo, and the effect on glucose metabolism was evaluated. RESULTS: GSK1904529A selectively inhibits IGF-IR and IR with IC(50)s of 27 and 25 nmol/L, respectively. GSK1904529A blocks receptor autophosphorylation and downstream signaling, leading to cell cycle arrest. It inhibits the proliferation of cell lines derived from solid and hematologic malignancies, with multiple myeloma and Ewing's sarcoma cell lines being most sensitive. Oral administration of GSK1904529A decreases the growth of human tumor xenografts in mice, consistent with a reduction of IGF-IR phosphorylation in tumors. Despite the potent inhibitory activity of GSK1904529A on IR in vitro and in vivo, minimal effects on blood glucose levels are observed in animals at doses that show significant antitumor activity. CONCLUSION: GSK1904529A is a promising candidate for therapeutic use in IGF-IR-dependent tumors.
GSK1904529A, an insulin-like growth factor-1 receptor inhibitor, inhibits glioma tumor growth, induces apoptosis and inhibits migration.[Pubmed:26035416]
Mol Med Rep. 2015 Sep;12(3):3381-3385.
Malignant gliomas are the most common type of primary malignancy of the central nervous system, with a poor prognosis. The therapeutic options for malignant gliomas are limited and far from satisfactory, and novel treatment strategies are urgently required to improve the outcome of the disease. Insulinlike growth factor (IGF)/IGF1 receptor (IGF1R) signaling pathway regulates cell proliferation, motility and survival. The dysregulation of this signaling pathway has been implicated in the development of malignant gliomas. In the present study, GSK1904529A, a small molecule inhibitor of IGF1R, suppressed glioma cell viability, induced glioma cell apoptosis and inhibited glioma cell migration in vitro. In addition, GSK1904529A inhibited glioma tumor growth and induced tumor cell apoptosis in vivo. In conclusion, the results of the present study suggested GSK1904529A as a promising agent for the treatment of malignant glioma.
GSK1904529A, a Potent IGF-IR Inhibitor, Reverses MRP1-Mediated Multidrug Resistance.[Pubmed:28266043]
J Cell Biochem. 2017 Oct;118(10):3260-3267.
Overexpression of multidrug-resistant efflux transporters is one of the major causes of chemotherapy failure. MRP1, a 190 kDa efflux transporter, confers resistance to a wide of range of chemotherapeutic drugs. Here we study the cellular effects of GSK1904529A in reversing MRP1-mediated drug resistance. Cytotoxicity of GSK1904529A was determined by MTT assay. Reversal effects of GSK1904529A in combination with MRP1 substrates were determined. The intracellular accumulation and efflux of MRP1 substrate was measured by scintillation counter and protein expression was determined by Western blotting analysis. Cell cycle effects of GSK1904529A in combination with MRP1 substrates were determined by flow cytometric analysis. GSK1904529A, at non-toxic concentrations, enhanced the cytotoxicity of MRP1 substrates in HEK293/MRP1 cells. Furthermore, GSK1904529A increased the intracellular accumulation of [(3) H]-vinblastine by inhibiting the efflux function of MRP1. GSK1904529A did not alter the expression level of MRP1, induced a G0/G1 phase cell cycle arrest. Our results indicated that GSK1904529A significantly increased the sensitivity of MRP1 overexpressing cells to chemotherapeutic agents. Furthermore, GSK1904529A enhanced the efficacy of chemotherapeutic drugs that are substrates of MRP1. J. Cell. Biochem. 118: 3260-3267, 2017. (c) 2017 Wiley Periodicals, Inc.


