ButylamineCAS# 109-73-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109-73-9 | SDF | Download SDF |
| PubChem ID | 8007 | Appearance | Powder |
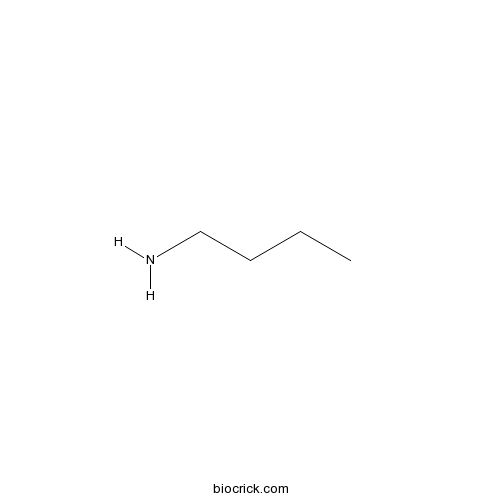
| Formula | C4H11N | M.Wt | 73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | butan-1-amine | ||
| SMILES | CCCCN | ||
| Standard InChIKey | HQABUPZFAYXKJW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H11N/c1-2-3-4-5/h2-5H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Butylamine Dilution Calculator

Butylamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 13.6986 mL | 68.4932 mL | 136.9863 mL | 273.9726 mL | 342.4658 mL |
| 5 mM | 2.7397 mL | 13.6986 mL | 27.3973 mL | 54.7945 mL | 68.4932 mL |
| 10 mM | 1.3699 mL | 6.8493 mL | 13.6986 mL | 27.3973 mL | 34.2466 mL |
| 50 mM | 0.274 mL | 1.3699 mL | 2.7397 mL | 5.4795 mL | 6.8493 mL |
| 100 mM | 0.137 mL | 0.6849 mL | 1.3699 mL | 2.7397 mL | 3.4247 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
Theoretical and Experimental Study on the Reaction of tert-Butylamine with OH Radicals in the Atmosphere.[Pubmed:29659281]
J Phys Chem A. 2018 May 10;122(18):4470-4480.
The OH-initiated atmospheric degradation of tert-Butylamine (tBA), (CH3)3CNH2, was investigated in a detailed quantum chemistry study and in laboratory experiments at the European Photoreactor (EUPHORE) in Spain. The reaction was found to mainly proceed via hydrogen abstraction from the amino group, which in the presence of nitrogen oxides (NO x), generates tert-butylnitramine, (CH3)3CNHNO2, and acetone as the main reaction products. Acetone is formed via the reaction of tert-butylnitrosamine, (CH3)3CNHNO, and/or its isomer tert-butylhydroxydiazene, (CH3)3CN horizontal lineNOH, with OH radicals, which yield nitrous oxide (N2O) and the (CH3)3C radical. The latter is converted to acetone and formaldehyde. Minor predicted and observed reaction products include formaldehyde, 2-methylpropene, acetamide and propan-2-imine. The reaction in the EUPHORE chamber was accompanied by strong particle formation which was induced by an acid-base reaction between photochemically formed nitric acid and the reagent amine. The tert-butylaminium nitrate salt was found to be of low volatility, with a vapor pressure of 5.1 x 10(-6) Pa at 298 K. The rate of reaction between tert-Butylamine and OH radicals was measured to be 8.4 (+/-1.7) x 10(-12) cm(3) molecule(-1) s(-1) at 305 +/- 2 K and 1015 +/- 1 hPa.


