Icariside B1CAS# 109062-00-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109062-00-2 | SDF | Download SDF |
| PubChem ID | 15628136 | Appearance | Oil |
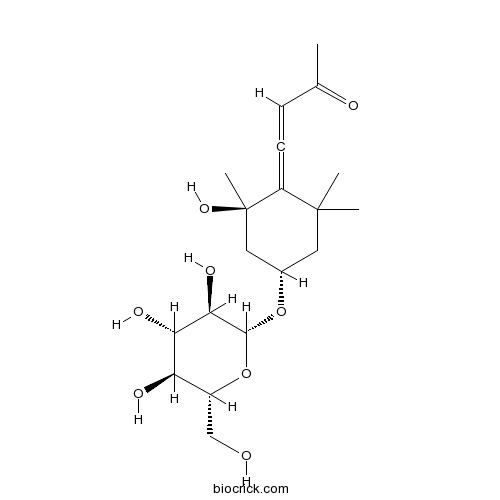
| Formula | C19H30O8 | M.Wt | 386.44 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(=O)C=C=C1C(CC(CC1(C)O)OC2C(C(C(C(O2)CO)O)O)O)(C)C | ||
| Standard InChIKey | OSUSRWMGHUUXBY-YARXSPMZSA-N | ||
| Standard InChI | InChI=1S/C19H30O8/c1-10(21)5-6-13-18(2,3)7-11(8-19(13,4)25)26-17-16(24)15(23)14(22)12(9-20)27-17/h5,11-12,14-17,20,22-25H,7-9H2,1-4H3/t6?,11-,12+,14+,15-,16+,17+,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Icariside B1 shows inhibitory effects on LPS-induced NO production in RAW264.7 cells. |
| Targets | NO | Akt |

Icariside B1 Dilution Calculator

Icariside B1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5877 mL | 12.9386 mL | 25.8772 mL | 51.7545 mL | 64.6931 mL |
| 5 mM | 0.5175 mL | 2.5877 mL | 5.1754 mL | 10.3509 mL | 12.9386 mL |
| 10 mM | 0.2588 mL | 1.2939 mL | 2.5877 mL | 5.1754 mL | 6.4693 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5175 mL | 1.0351 mL | 1.2939 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5175 mL | 0.6469 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- Mauritianin
Catalog No.:BCN2932
CAS No.:109008-28-8
- Ilexoside D
Catalog No.:BCN7865
CAS No.:109008-27-7
- Ilexoside K
Catalog No.:BCN7866
CAS No.:109008-26-6
- 2-Methylaminoethanol
Catalog No.:BCN1758
CAS No.:109-83-1
- Butylamine
Catalog No.:BCC8304
CAS No.:109-73-9
- Allylthiourea
Catalog No.:BCC4759
CAS No.:109-57-9
- N-Valeric acid
Catalog No.:BCC8220
CAS No.:109-52-4
- FURA-2AM
Catalog No.:BCC7296
CAS No.:108964-32-5
- Neocurdione
Catalog No.:BCC9242
CAS No.:108944-67-8
- H-7 dihydrochloride
Catalog No.:BCC6686
CAS No.:108930-17-2
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- 3Beta-Isodihydrocadambine 4-oxide
Catalog No.:BCN3651
CAS No.:1092371-18-0
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
Chemical constituents of the Annona glabra fruit and their cytotoxic activity.[Pubmed:25856711]
Pharm Biol. 2015;53(11):1602-7.
CONTEXT: Traditional Chinese medicines have attracted increasing interest as potential sources of novel drugs with a wide range of biological and pharmacological activities. Annona glabra Linn (Annonaceae) is used in traditional medicine as an anticancer drug. Phytochemical investigation of this plant led to the isolation of acetogenins, ent-kauranes, peptides, and alkaloids. In addition, compounds exhibited anticancer, anti-HIV-reserve, and antimalaria. OBJECTIVE: Isolation, structure determination, and cytotoxic activity evaluation of compounds from the methanol extract from A. glabra fruits. MATERIALS AND METHODS: Using chromatographic methods to isolate compounds from the A. glabra methanol extract. The cytotoxic activity of compounds was evaluated by a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. In addition, compounds which showed significant cytotoxic activity were chosen for further study apoptosis characteristics. RESULTS: One new, (2E,4E,1'R,3'S,5'R,6'S)-dihydrophaseic acid 1,3'-di-O-beta-d-glucopyranoside, and eight known compounds, (2E,4E,1'R,3'S,5'R,6'S)-dihydrophaseic acid 3'-O-beta-d-glucopyranoside (2), icariside D2 (3), icariside D2 6'-O-beta-d-xylopyranoside (4), 3,4-dimethoxyphenyl O-beta-d-glucopyranoside (5), 3,4-dihydroxybenzoic acid (6), blumenol A (7), cucumegastigmane I (8), and Icariside B1 (9), were isolated from the fruits of A. glabra. Icariside D2 (3) was found to show significant cytotoxic activity on the HL-60 cell line with the IC50 value of 9.0 +/- 1.0 microM and did not show cytotoxic activity on the Hel-299 normal cell line. The further test indicated that compound 3 induced apoptosis via alteration of expression of apoptosis-related proteins and decreased phosphorylation of AKT in HL-60 cells. DISCUSSION AND CONCLUSION: The results suggested that the constituents from A. glabra may contain effective compounds which can be used as anticancer agents.
Two new sulfated sesquiterpenoids from Petasites tricholobus.[Pubmed:25577874]
Yao Xue Xue Bao. 2014 Oct;49(10):1433-7.
Two new sulfated sesquiterpenoids, megastigman-7-ene-3, 5, 6, 9-tetrol-3-O-beta-D-6'-sulfonated-glucopyranoside (1) and 3-O-beta-D-6'-sulfonated-glucopyranosyl-6-(3-oxo-2-butenylidenyl)-1, 1, 5-trimethylcyclohexan-5-ol (2), along with one known sesquitepenoid compound Icariside B1 (3) were isolated from the whole herb of Petasites tricholobus Franch. Their structures were identified by their chemical and spectroscopic characters. All obtained compounds were tested for their cytotoxicity against four cancer cell lines.
Triterpene saponins and megastigmane glucosides from Camellia bugiamapensis.[Pubmed:28011215]
Bioorg Med Chem Lett. 2017 Feb 1;27(3):557-561.
Two new triterpene saponins, camelliosides I and J (1 and 2), two new megastigmane glycosides, camellistigosides A and B (3 and 4), and two known megastigmane glycosides, Icariside B1 (5) and (6S,9R)-roseoside (6), were isolated from a methanol extract of the Camellia bugiamapensis leaves using various chromatographic separation techniques. Their structures were elucidated based on spectroscopic analyses, including HR ESI MS, CD, 1D and 2D NMR. Their inhibitory effects on LPS-induced NO production in RAW264.7 cells were evaluated. This is the first report of the chemical constituents and biological activity of C. bugiamapensis.


