IsomeranzinCAS# 1088-17-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1088-17-1 | SDF | Download SDF |
| PubChem ID | 473252 | Appearance | Powder |
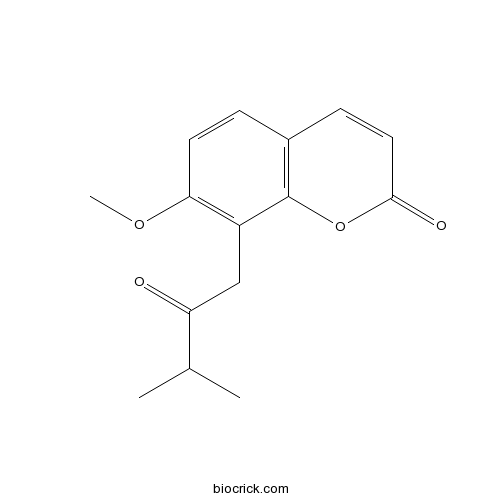
| Formula | C15H16O4 | M.Wt | 260.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-methoxy-8-(3-methyl-2-oxobutyl)chromen-2-one | ||
| SMILES | CC(C)C(=O)CC1=C(C=CC2=C1OC(=O)C=C2)OC | ||
| Standard InChIKey | OMOYQLRHKFGVGN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H16O4/c1-9(2)12(16)8-11-13(18-3)6-4-10-5-7-14(17)19-15(10)11/h4-7,9H,8H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isomeranzin has anti-inflammatory activity, it suppresses inflammation by inhibiting M1 macrophage polarization through the p65, NF-κB and ERK pathway. |
| Targets | NF-kB | ERK |
| In vivo | Isomeranzin suppresses inflammation by inhibiting M1 macrophage polarization through the NF-κB and ERK pathway.[Pubmed: 27285671 ]Int Immunopharmacol. 2016 Sep;38:175-85.Macrophage polarization plays an important role in inflammation. Regulation of the polarization has been reported to be effective therapeutics for various kinds of inflammatory diseases. The aims of the present study were to investigate the anti-inflammatory property of Isomeranzin isolating from Murraya exotica as well as potential molecular mechanisms.
|

Isomeranzin Dilution Calculator

Isomeranzin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8417 mL | 19.2086 mL | 38.4172 mL | 76.8344 mL | 96.043 mL |
| 5 mM | 0.7683 mL | 3.8417 mL | 7.6834 mL | 15.3669 mL | 19.2086 mL |
| 10 mM | 0.3842 mL | 1.9209 mL | 3.8417 mL | 7.6834 mL | 9.6043 mL |
| 50 mM | 0.0768 mL | 0.3842 mL | 0.7683 mL | 1.5367 mL | 1.9209 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7683 mL | 0.9604 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- Mizolastine
Catalog No.:BCC4521
CAS No.:108612-45-9
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- Lumichrome
Catalog No.:BCN7083
CAS No.:1086-80-2
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- Ilexgenin A
Catalog No.:BCC9233
CAS No.:108524-94-3
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
- GSK-923295
Catalog No.:BCC1608
CAS No.:1088965-37-0
- MK 6096
Catalog No.:BCC4020
CAS No.:1088991-73-4
- Soyasaponin IV
Catalog No.:BCN1627
CAS No.:108906-97-4
- U0124
Catalog No.:BCC7200
CAS No.:108923-79-1
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
Antimycobacterial activity of chemically defined natural substances from the Caribbean flora in Guadeloupe.[Pubmed:9626931]
FEMS Immunol Med Microbiol. 1998 Apr;20(4):267-73.
Eight chemically defined, naturally occurring compounds were extracted from the tropical flora of the Caribbean island of Guadeloupe: pilocarpine, an alkaloid from Pilocarpus racemosus; heraclenol and Isomeranzin, coumarins from Triphasia trifolia; lochnerin, an indole alkaloid from Rauwolfia biauriculata; ibogaine and voacangine, indole alkaloids from Tabernaemontana citrifolia; texalin, an oxazole from Amyris elemifera; and canellal, a sesquiterpene dialdehyde from Canella winterana. An essential oil fraction from Canella winterana was also tested. The antimycobacterial activity of these substances was tested against Mycobacterium tuberculosis, M. avium and M. kansasii using the Middlebrook 7H11 agar medium, the Bactec 460-TB radiometric methodology, and determination of bacterial viable counts. Three compounds, namely ibogaine, voacangine and texalin, showed antimycobacterial activity. Investigations on the structure-modification and structure-activity relationships of these compounds may help determine new targets for future drug development.


