MizolastineCAS# 108612-45-9 |
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Proflavine Hemisulfate
Catalog No.:BCC4707
CAS No.:1811-28-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- NSC 146109 hydrochloride
Catalog No.:BCC2410
CAS No.:59474-01-0
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108612-45-9 | SDF | Download SDF |
| PubChem ID | 65906 | Appearance | Powder |
| Formula | C24H25FN6O | M.Wt | 432.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (57.80 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2-[[1-[1-[(4-fluorophenyl)methyl]benzimidazol-2-yl]piperidin-4-yl]-methylamino]-1H-pyrimidin-6-one | ||
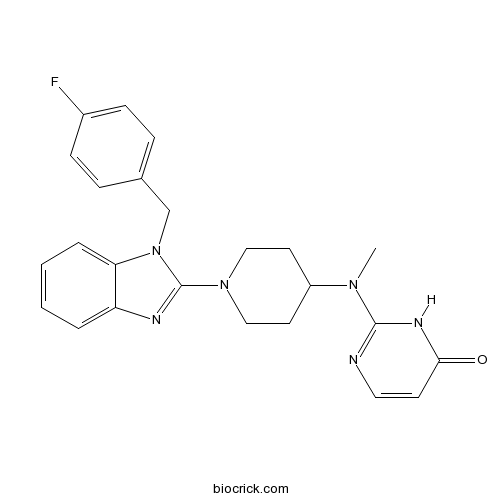
| SMILES | CN(C1CCN(CC1)C2=NC3=CC=CC=C3N2CC4=CC=C(C=C4)F)C5=NC=CC(=O)N5 | ||
| Standard InChIKey | PVLJETXTTWAYEW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mizolastine Dilution Calculator

Mizolastine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3122 mL | 11.561 mL | 23.1219 mL | 46.2438 mL | 57.8048 mL |
| 5 mM | 0.4624 mL | 2.3122 mL | 4.6244 mL | 9.2488 mL | 11.561 mL |
| 10 mM | 0.2312 mL | 1.1561 mL | 2.3122 mL | 4.6244 mL | 5.7805 mL |
| 50 mM | 0.0462 mL | 0.2312 mL | 0.4624 mL | 0.9249 mL | 1.1561 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2312 mL | 0.4624 mL | 0.578 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mizolastine is a histamine H1-receptor antagonist with IC50 of 47 nM used in the treatment of hay fever (seasonal allergic rhinitis), hives and other allergic reactions.
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- Lumichrome
Catalog No.:BCN7083
CAS No.:1086-80-2
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- Ilexgenin A
Catalog No.:BCC9233
CAS No.:108524-94-3
- Ilexsaponin A
Catalog No.:BCN7867
CAS No.:108524-93-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- Fuligorubin A
Catalog No.:BCN1837
CAS No.:108343-55-1
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
- Taccalonolide A
Catalog No.:BCN2737
CAS No.:108885-68-3
- Taccalonolide B
Catalog No.:BCN2743
CAS No.:108885-69-4
- 13-O-Acetylcorianin
Catalog No.:BCN5883
CAS No.:108887-44-1
Development of a CZE method for the determination of mizolastine and its impurities in pharmaceutical preparations using response surface methodology.[Pubmed:17195261]
Electrophoresis. 2007 Feb;28(3):395-405.
A fast and selective CZE method for the determination of Mizolastine and related impurities is described. Response surface methodology was applied to study the influence of phosphate/triethanolamine (TEA) buffer concentration, heptakis(2,3,6-tri-O-methyl)-beta-CD (TMbetaCD) concentration, voltage and temperature. The optimum conditions were: 105 mM phosphate/TEA buffer (pH 3.0) containing 10 mM TMbetaCD, temperature 19 degrees C and voltage 30 kV. Validation of the method was performed in drug substance and drug product. Robustness was evaluated using a Plackett-Burman design, including pH among the considered factors. Applying the optimal conditions, the nine peaks were baseline separated in about 10 min. The method was applied to the quality control of Mizolastine in controlled-release tablets.
[Efficacy and safety of Mizolastine in the treatment of perennial allergic rhinitis].[Pubmed:17674763]
Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007 Jun;21(11):491-3.
OBJECTIVE: To investigate the efficacy and safety of Mizolastine in the treatment of perennial allergic rhinitis. METHOD: Multicentric random Double-blind parallel-controlled study was adopted, and compared with placebo and Cetirizine. Patients (n = 177) were grouped, seventy-two in Mizolastine group, sixty-nine in Cetirizine and thirty-six in placebo group. RESULT: In the seventh curative day symptomatic and sign marks in Mizolastine group and Cetirizine group were lower, but the mark in Mizolastine group reduced more than in Cetirizine group and placebo group. Mizolastine group is better than Cetirizine group in improvement of nasal obstruction and itching with Visual analogue scale. In the twenty first curative day reduction of symptomatic and sign marks in Mizolastine group was lower than Cetirizine group, but no statistic difference. There were 27 adverse events, no serious adverse events in 177 patients during experimental period. Most adverse events were headache and dryness in mouth and eyes. There were 10 cases adverse events in Mizolastine group, one case was related with experiment and four cases might be related with experiment. There were 14 cases adverse events in Cetirizine group, one case was related with experiment and four cases might be related with experiment. There were three cases adverse events in placebo group. CONCLUSION: Generally speaking the efficacy of Mizolastine in treatment of perennial allergic rhinitis is better than Cetirizine, Bad events are less. It is safe.
Mizolastine: a review of its use in allergic rhinitis and chronic idiopathic urticaria.[Pubmed:18020585]
BioDrugs. 1998 Jul;10(1):41-63.
UNLABELLED: Mizolastine is a second generation antihistamine agent with high affinity and specificity for histamine H(1) receptors. Mizolastine has demonstrated antiallergic effects in animals and healthy volunteers and anti-inflammatory activity in animal models. Double-blind trials have shown Mizolastine to be significantly more effective than placebo and as effective as other second generation antihistamine agents, such as loratadine or cetirizine, in the management of patients with perennial or seasonal allergic rhinitis and in patients with chronic idiopathic urticaria. Available data also suggest that prophylactic administration of Mizolastine is significantly more effective than placebo and as effective as prophylactic terfenadine in delaying the onset of symptoms of seasonal allergic rhinitis. Mizolastine 10 mg/day is generally well tolerated, with the most common adverse events being drowsiness (7%), fatigue (2%), increased appetite (2%) and dry mouth (2%). In volunteers and patients the incidence of prolonged QT(c) interval was similar in Mizolastine and placebo recipients, although Mizolastine is contraindicated in those with cardiac disease or hepatic impairment or in those receiving erythromycin, ketoconazole or class I or III antiarrhythmic agents. Tests of psychomotor function in volunteers revealed no impairment after single doses of Mizolastine
Reversible anti-settlement activity against Amphibalanus (=Balanus) amphitrite, Bugula neritina, and Hydroides elegans by a nontoxic pharmaceutical compound, mizolastine.[Pubmed:20183132]
Biofouling. 2009 Nov;25(8):739-47.
Mizolastine, an antihistamine pharmaceutical, was found to significantly inhibit larval settlement of the barnacle Amphibalanus (=Balanus) amphitrite, the bryozoan Bugula neritina, and the polychaete Hydroides elegans with EC(50) values of 4.2, 11.2, and 4.1 microg ml(-1), respectively. No toxicity against the larvae of these three species was observed at the concentration range tested during incubations with Mizolastine. To determine whether the anti-settlement activity of Mizolastine is reversible, recovery bioassays using these three species were conducted. More than 70% of the larvae that had been exposed for 4 h to Mizolastine at concentrations four-fold greater than their respective EC(50) values completed normal metamorphosis. The results of the recovery bioassay provide evidence that the anti-settlement effect of Mizolastine is reversible in addition to being nontoxic. The anti-settlement activities of several intermediates of the synthesis process of Mizolastine were also examined. One of the intermediates, 2-chloro-1-(4-fluorobenzyl)-1H-benzo[d]imidazole, inhibited larval settlement and metamorphosis with low toxicity. These results may improve the understanding of the key functional group responsible for the anti-settlement activity of Mizolastine.


