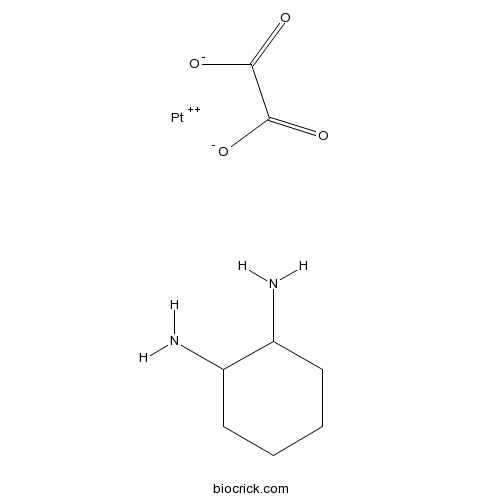
OxaliplatinAntitumor agent CAS# 61825-94-3 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61825-94-3 | SDF | Download SDF |
| PubChem ID | 77994 | Appearance | Powder |
| Formula | C8H14N2O4Pt | M.Wt | 397.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Eloxatin | ||
| Solubility | DMF : 4 mg/mL (10.07 mM; Need ultrasonic; DMSO can inactivate Oxaliplatin's activity) H2O : 1.7 mg/mL (4.28 mM; Need ultrasonic and warming; DMSO can inactivate Oxaliplatin's activity) | ||
| Chemical Name | cyclohexane-1,2-diamine;oxalate;platinum(2+) | ||
| SMILES | C1CCC(C(C1)N)N.C(=O)(C(=O)[O-])[O-].[Pt+2] | ||
| Standard InChIKey | ZROHGHOFXNOHSO-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C6H14N2.C2H2O4.Pt/c7-5-3-1-2-4-6(5)8;3-1(4)2(5)6;/h5-6H,1-4,7-8H2;(H,3,4)(H,5,6);/q;;+2/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antitumor agent that forms platinum-DNA adducts. Causes intra- and interstrand DNA crosslinks blocking DNA replication and transcription. Displays higher cytotoxicity and lower nephrotoxicity than analog cisplatin and shows antitumor activity in cell lines with acquired cisplatin resistance. |

Oxaliplatin Dilution Calculator

Oxaliplatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5171 mL | 12.5853 mL | 25.1705 mL | 50.3411 mL | 62.9263 mL |
| 5 mM | 0.5034 mL | 2.5171 mL | 5.0341 mL | 10.0682 mL | 12.5853 mL |
| 10 mM | 0.2517 mL | 1.2585 mL | 2.5171 mL | 5.0341 mL | 6.2926 mL |
| 50 mM | 0.0503 mL | 0.2517 mL | 0.5034 mL | 1.0068 mL | 1.2585 mL |
| 100 mM | 0.0252 mL | 0.1259 mL | 0.2517 mL | 0.5034 mL | 0.6293 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Antitumor agent that forms platinum-DNA adducts. Causes intra- and interstrand DNA crosslinks blocking DNA replication and transcription. Displays higher cytotoxicity and lower nephrotoxicity than analog cisplatin and shows antitumor activity in cell line
- Trans-Melilotoside
Catalog No.:BCC8364
CAS No.:618-67-7
- Ethyl 3,4,5-trimethoxybenzoate
Catalog No.:BCN3973
CAS No.:6178-44-5
- 5-O-Methylnaringenin
Catalog No.:BCN4144
CAS No.:61775-19-7
- Methyl 2-(6-acetyl-5-hydroxy-2,3-dihydrobenzofuran-2-yl)propenoate
Catalog No.:BCN1395
CAS No.:617722-56-2
- Methyl 2-(5-acetyl-2,3-dihydrobenzofuran-2-yl)propenoate
Catalog No.:BCN1396
CAS No.:617722-55-1
- Fluvoxamine maleate
Catalog No.:BCC1215
CAS No.:61718-82-9
- W-7 hydrochloride
Catalog No.:BCC6622
CAS No.:61714-27-0
- W-5 hydrochloride
Catalog No.:BCC6621
CAS No.:61714-25-8
- 2-Chloro-1,4-phenylenediamine sulfate
Catalog No.:BCN8435
CAS No.:61702-44-1
- 2-Hydroxy-1-methoxyanthraquinone
Catalog No.:BCN3091
CAS No.:6170-06-5
- Ethyl vanillate
Catalog No.:BCN3670
CAS No.:617-05-0
- OMDM-2
Catalog No.:BCC5814
CAS No.:616884-63-0
- Sipeimine
Catalog No.:BCN1201
CAS No.:61825-98-7
- Vorapaxar
Catalog No.:BCC3996
CAS No.:618385-01-6
- Benzyl p-coumarate
Catalog No.:BCN7717
CAS No.:61844-62-0
- Epoprostenol
Catalog No.:BCC7534
CAS No.:61849-14-7
- Demethoxycapillarisin
Catalog No.:BCN4611
CAS No.:61854-36-2
- Demethoxy-7-O-methylcapillarisin
Catalog No.:BCN6469
CAS No.:61854-37-3
- H-β-homo-Gln-OH.HCl
Catalog No.:BCC2648
CAS No.:61884-74-0
- Vernolide B
Catalog No.:BCN6749
CAS No.:618860-58-5
- 3-(1,1-Dimethylallyl)-8-hydroxy-7-methoxycoumarin
Catalog No.:BCN7570
CAS No.:61899-42-1
- 4-Hydroxybenzamide
Catalog No.:BCN4147
CAS No.:619-57-8
- p-Ethoxybenzoic acid
Catalog No.:BCN3378
CAS No.:619-86-3
- 4-Nitrocinnamic acid
Catalog No.:BCN5033
CAS No.:619-89-6
Combined effects off indomethacin and oxaliplatin on lymph node metastasis related factors in human lung cancerxenografts in nude mice.[Pubmed:28375128]
Pak J Pharm Sci. 2016 Nov;29(6):2083-2088.
To investigate the combined effects of indomethacin and Oxaliplatin on expressions of epidermal growth factor receptor (EGFR), E-cadherin (E-cad), intercellular adhesion molecule-1 (ICAM-1) and CD44v6 related to lymph node metastasis of human lung cancer cell lines. Human lung adenocarcinoma A549 cells were inoculated subcutaneously into the left armpit of nude mice to establish human lung cancer xenografts. The mice were randomly divided into control group, indomethacin group, Oxaliplatin group and combination therapy group, which were treated with sterile distilled water, indomethacin, Oxaliplatin and indomethacin combined with Oxaliplatin, respectively. After 42 days, the mice were sacrificed. The immunohistochemistry and reverse transcription polymerase chain reaction were used to detect the expressions of EGFR, E-cad, ICAM-1 and CD44v6 in tumor tissues. Compared to control group, the protein and mRNA expressions of EGFR, ICAM-1 and CD44v6 in the indomethacin, Oxaliplatin, and combination therapy groups were significantly reduced (P<0.05) and the protein and mRNA expressions of E-cad expression were significantly increased (P<0.05). Compared to indomethacin group and Oxaliplatin group, the protein and mRNA expressions of EGFR, ICAM-1 and CD44v6 in combination therapy groups were significantly reduced (P<0.05), and the protein and mRNA expressions of E-cad expression were significantly increased (P<0.05). There was no significant difference between indomethacin and Oxaliplatin groups. Indomethacin and Oxaliplatin have synergistic effect on expressions of lymph node metastasis related factors in lung cancer cell lines.
Autophagy impacts on oxaliplatin-induced hepatocarcinoma apoptosis via the IL-17/IL-17R-JAK2/STAT3 signaling pathway.[Pubmed:28356957]
Oncol Lett. 2017 Feb;13(2):770-776.
The interleukin (IL)-17/IL-17 receptor (IL-17R) complex has been shown to be important for the regulation of inflammation; however, its role in the regulation of tumor processes has recently emerged as a research focus. The present study demonstrated that Oxaliplatin was able to increase the levels of IL-17/IL-17R in hepatocellular carcinoma (HCC) patients and cells lines, and that it had important roles in reducing the susceptibility of the cells to Oxaliplatin-induced apoptosis. Furthermore, the expression of autophagy-related proteins was induced by IL-17/IL-17R and autophagy was shown to induce resistance to Oxaliplatin in HCC. In addition, the janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway was shown to be an important pathway in the induction of autophagy in response to Oxaliplatin. Autopjhagy was inhibited by 3-methyladenine and JAK2/STAT3 signaling was blocked by AG490, which induced apoptosis in SMMC7721 cells treated with Oxaliplatin. The results of the present study may help to elucidate the mechanism underlying the role of IL-17/IL-17R-induced autophagy in the chemoresistance of HCC, as well as help to establish and develop measures to overcome chemoresistance in HCC.
Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis.[Pubmed:28373752]
Chin J Cancer Res. 2017 Feb;29(1):36-44.
OBJECTIVE: To investigate the prognostic factors in chemorefractory colorectal cancer liver metastasis (CRCLM) patients treated by transarterial chemoembolization (TACE) and sustained hepatic arterial infusion chemotherapy (HAIC). METHODS: Between 2006 and 2015, 162 patients who underwent 763 TACE and HAIC in total were enrolled in this retrospective study, including 110 males and 52 females, with a median age of 60 (range, 26-83) years. Prognostic factors were assessed with Log-rank test, Cox univariate and multivariate analyses. RESULTS: The median survival time (MST) and median progression-free survival (PFS) of the 162 patients from first TACE/HAIC were 15.6 months and 5.5 months respectively. Normal serum carbohydrate antigen 19-9 (CA19-9, <37 U/mL) (P<0.001) and carbohydrate antigen 72-4 (CA72-4, <6.7 U/mL) (P=0.026), combination with other local treatment (liver radiotherapy or liver radiofrequency ablation) (P=0.034) and response to TACE/HAIC (P<0.001) were significant factors related to survival after TACE/HAIC in univariate analysis. A multivariate analysis revealed that normal serum CA19-9 (P<0.001), response to TACE/HAIC (P<0.001) and combination with other local treatment (P=0.001) were independent factors among them. CONCLUSIONS: Our findings indicate that serum CA19-9 <37 U/mL and response to TACE/HAIC are significant prognostic indicators for this combined treatment, and treated with other local treatment could reach a considerable survival benefit for CRCLM. This could be useful for making decisions regarding the treatment of CRCLM.
Multicenter phase II study of infusional 5-fluorouracil (5-FU), leucovorin, and oxaliplatin, plus biweekly cetuximab as first-line treatment in patients with metastatic colorectal cancer (CELINE trial).[Pubmed:28356954]
Oncol Lett. 2017 Feb;13(2):747-753.
The current phase II study investigated the efficacy and safety of biweekly cetuximab combined with standard Oxaliplatin-based chemotherapy [infusional 5-fluorouracil (5-FU), leucovorin, and Oxaliplatin (FOLFOX-6)] in the first-line treatment of KRAS wild-type metastatic colorectal cancer (mCRC). Sixty patients with a median age of 64 years (range, 38-82 syears) received a biweekly intravenous infusion of cetuximab (500 mg/m(2) on day 1) followed by FOLFOX-6 (2-hour Oxaliplatin 85 mg/m(2) infusion on day 1 in tandem with a 2-h leucovorin 200 mg/m(2) infusion on days 1 and 2, and 5-FU as a 400 mg/m(2) bolus followed by a 46-hour 2,400 mg/m(2) infusion on days 1-3). Patient response rate was 70%, with 95% disease control rates. The median progression-free survival was 13.8 months. Thirteen patients (21.7%) were able to undergo resection of previously unresectable metastases, with the aim of curing them. The median follow-up was 22.7 months, and median overall survival was 31.0 months. Cetuximab did not increase FOLFOX-6 toxicity and was generally well tolerated. The results of the current study demonstrate that the combination of biweekly cetuximab with FOLFOX-6 was well tolerated and had a manageable safety profile for the first-line treatment of KRAS wild-type metastatic colorectal cancer. Efficacy was comparable to other treatment regimens. The results support the administration of biweekly cetuximab in combination with FOLFOX-6, which may be more convenient and provide treatment flexibility in this setting for patients with metastatic colorectal cancers.
Cellular and molecular pharmacology of oxaliplatin.[Pubmed:12467217]
Mol Cancer Ther. 2002 Jan;1(3):227-35.
Oxaliplatin, a diaminocyclohexane-containing platinum, has a spectrum of activity and mechanisms of action and resistance that appear to be different from those of other platinum-containing compounds, notably cisplatin. The first part of this review describes the differences between Oxaliplatin and cisplatin in terms of their spectrum of activity and adduct formation and then goes on to discuss molecular and cellular experimental data that potentially explain them. Particular emphasis is placed on the differential role of DNA repair mechanisms. In addition, the anticancer effects of Oxaliplatin are optimized when it is administered in combination with other anticancer agents, such as 5-fluorouracil, gemcitabine, cisplatin, or carboplatin; topoisomerase I inhibitors; and taxanes. In vitro and preclinical combination data that could optimize Oxaliplatin-based chemotherapy are also reviewed.
Oxaliplatin. A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies.[Pubmed:11085200]
Drugs. 2000 Oct;60(4):895-924.
UNLABELLED: Oxaliplatin is a platinum compound that inhibits DNA synthesis, primarily by causing intrastrand cross-links in DNA. Oxaliplatin has a broad spectrum of antineoplastic activity and has demonstrated a lack of cross-resistance with other platinum compounds. In patients with metastatic colorectal cancer, intravenous Oxaliplatin has been trialled as a monotherapy and in combination with other agents. The highest response rates were achieved when Oxaliplatin was used in combination with fluorouracil/folinic acid (leucovorin; calcium folinate), typically > or = 50% in the first-line setting and 13 to 45% as a second-line therapy. First-line triple therapy with Oxaliplatin and fuorouracil/folinic acid achieved significantly higher response rates and longer median progression-free survival than fluorouracil/folinic acid therapy alone. However, no significant difference in the median duration of overall survival was found. This may be a consequence of the subsequent use of Oxaliplatin and/or surgery after disease progression in patients who relapsed after fluorouracil/folinic acid therapy alone. Neoadjuvant therapy with Oxaliplatin/fluorouracil/folinic acid has proven beneficial in enabling surgical removal of previously unresectable liver metastases. In 2 studies, surgery with curative intent was performed in 16 and 51% of patients with initially unresectable liver metastases following Oxaliplatin/fluorouracil/folinic acid therapy; the 5-year survival rates were 40 and 50%, respectively. In patients with advanced ovarian cancer, first-line therapy with Oxaliplatin/cyclophosphamide achieved an objective response rate which did not differ significantly from that of cisplatin/cyclophosphamide (33 vs 42%). In addition, Oxaliplatin has shown efficacy in patients with platinum-pretreated ovarian cancer and achieved objective response rates similar to paclitaxel in this setting (16 vs 17%). Promising results have also been found with Oxaliplatin in patients with non-Hodgkin's lymphoma, breast cancer, mesothelioma and non-small cell lung cancer. Reversible, cumulative, peripheral sensory neuropathy is the principle dose-limiting factor of Oxaliplatin therapy. Haematological and gastrointestinal toxicities occur frequently but are generally mild to moderate in intensity. CONCLUSION: Oxaliplatin in combination with fluorouracil/folinic acid is an effective treatment option for patients with metastatic colorectal cancer, both as a first-line therapy and in patients refractory to previous chemotherapy. Although preliminary results failed to show any overall survival advantage of this regimen over fluorouracil/folinic acid alone, this may be a consequence of trial design and requires further examination. Additional clinical investigation of Oxaliplatin in patients with other cancers is warranted given the promising results achieved in early trials, most notably in patients with platinum-pretreated ovarian cancer.


