OMDM-2Potent inhibitor of anandamide uptake CAS# 616884-63-0 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 616884-63-0 | SDF | Download SDF |
| PubChem ID | 73755147 | Appearance | Powder |
| Formula | C27H45NO3 | M.Wt | 431.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in ethanol and to 10 mM in DMSO | ||
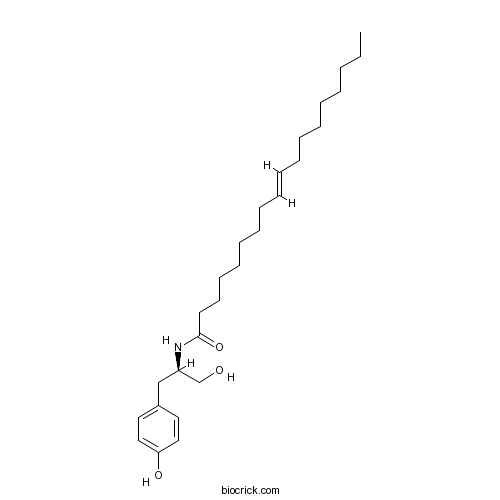
| Chemical Name | (E)-N-[(2R)-1-hydroxy-3-(4-hydroxyphenyl)propan-2-yl]octadec-9-enamide | ||
| SMILES | CCCCCCCCC=CCCCCCCCC(=O)NC(CC1=CC=C(C=C1)O)CO | ||
| Standard InChIKey | ICDMLAQPOAVWNH-YHZGQTRVSA-N | ||
| Standard InChI | InChI=1S/C27H45NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-27(31)28-25(23-29)22-24-18-20-26(30)21-19-24/h9-10,18-21,25,29-30H,2-8,11-17,22-23H2,1H3,(H,28,31)/b10-9+/t25-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Metabolically stable inhibitor of anandamide cellular uptake (Ki = 3 μM). Displays relatively low affinity for CB1 and CB2 receptors (Ki values are 5.1 and > 10 μM) and for vanilloid VR1 receptors (EC50 = 10 μM). Has minimal activity against FAAH (Ki > 50 μM). Active in vivo. |

OMDM-2 Dilution Calculator

OMDM-2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3166 mL | 11.5832 mL | 23.1664 mL | 46.3328 mL | 57.916 mL |
| 5 mM | 0.4633 mL | 2.3166 mL | 4.6333 mL | 9.2666 mL | 11.5832 mL |
| 10 mM | 0.2317 mL | 1.1583 mL | 2.3166 mL | 4.6333 mL | 5.7916 mL |
| 50 mM | 0.0463 mL | 0.2317 mL | 0.4633 mL | 0.9267 mL | 1.1583 mL |
| 100 mM | 0.0232 mL | 0.1158 mL | 0.2317 mL | 0.4633 mL | 0.5792 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Alaninol
Catalog No.:BCC2731
CAS No.:6168-72-5
- 3-(Dimethylsulfonio)-N,N,N-trimethylpropanaminium(2+)
Catalog No.:BCN1397
CAS No.:61672-51-3
- Trimethyl[3-(methylthio)propyl]ammonium(1+)
Catalog No.:BCN1398
CAS No.:61672-50-2
- Neridienone B
Catalog No.:BCN4143
CAS No.:61671-56-5
- 11-Methoxyuncarine C
Catalog No.:BCN4142
CAS No.:61665-08-5
- Furomollugin
Catalog No.:BCN4141
CAS No.:61658-41-1
- MK 212 hydrochloride
Catalog No.:BCC6856
CAS No.:61655-58-1
- Propargyl p-toluenesulfonate
Catalog No.:BCN2266
CAS No.:6165-76-0
- Propargyl benzenesulfonate
Catalog No.:BCN2247
CAS No.:6165-75-9
- Protopine hydrochloride
Catalog No.:BCN5345
CAS No.:6164-47-2
- Amfenac Sodium Monohydrate
Catalog No.:BCC4620
CAS No.:61618-27-7
- Songoroside A
Catalog No.:BCN3988
CAS No.:61617-29-6
- Ethyl vanillate
Catalog No.:BCN3670
CAS No.:617-05-0
- 2-Hydroxy-1-methoxyanthraquinone
Catalog No.:BCN3091
CAS No.:6170-06-5
- 2-Chloro-1,4-phenylenediamine sulfate
Catalog No.:BCN8435
CAS No.:61702-44-1
- W-5 hydrochloride
Catalog No.:BCC6621
CAS No.:61714-25-8
- W-7 hydrochloride
Catalog No.:BCC6622
CAS No.:61714-27-0
- Fluvoxamine maleate
Catalog No.:BCC1215
CAS No.:61718-82-9
- Methyl 2-(5-acetyl-2,3-dihydrobenzofuran-2-yl)propenoate
Catalog No.:BCN1396
CAS No.:617722-55-1
- Methyl 2-(6-acetyl-5-hydroxy-2,3-dihydrobenzofuran-2-yl)propenoate
Catalog No.:BCN1395
CAS No.:617722-56-2
- 5-O-Methylnaringenin
Catalog No.:BCN4144
CAS No.:61775-19-7
- Ethyl 3,4,5-trimethoxybenzoate
Catalog No.:BCN3973
CAS No.:6178-44-5
- Trans-Melilotoside
Catalog No.:BCC8364
CAS No.:618-67-7
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
Activity-based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2.[Pubmed:20471226]
Eur Neuropsychopharmacol. 2010 Sep;20(9):622-31.
The activity-based anorexia (ABA) paradigm is one of the few animal models of human anorexia nervosa. We present here the translation of this approach to C57/BL6 mice, a common background for genetically modified mice, and investigate the effects of the cannabinoid agonist, Delta(9)-tetrahydrocannabinol (THC) and the endocannabinoid uptake inhibitor, OMDM-2 in this model. The ABA paradigm was optimised so that food-restricted wheel-running mice displayed anorexia, reduced body weight and disrupted activity and circadian cycles. These conditions produced a murine ABA model with a defined stage and stability to allow for pharmacological intervention. Daily Delta(9)-THC (0.5 mg/kg) decreased survival in the ABA animals but increased feeding in the survivors, OMDM-2 (3 mg/kg) increased food intake, but not sufficiently to reverse weight loss. The effects of this model on endocannabinoid tone in the brain remain to be determined. Since the endocannabinoid system may be implicated in anorexia nervosa and in view of the positive modulation by cannabinoids of some aspects of ABA in this study, further investigation of the effects of cannabinoids in ABA is warranted.
The administration of endocannabinoid uptake inhibitors OMDM-2 or VDM-11 promotes sleep and decreases extracellular levels of dopamine in rats.[Pubmed:23238438]
Physiol Behav. 2013 Jan 17;109:88-95.
The family of the endocannabinoid system comprises endogenous lipids (such as anandamide [ANA]), receptors (CB(1)/CB(2) cannabinoid receptors), metabolic enzymes (fatty acid amide hydrolase [FAAH]) and a putative membrane transporter (anandamide membrane transporter [AMT]). Although the role of ANA, FAAH or the CB(1) cannabinoid receptor in sleep modulation has been reported, the effects of the inhibition of AMT on sleep remain unclear. In the present study, we show that microdialysis perfusion in rats of AMT inhibitors, (9Z)-N-[1-((R)-4-hydroxbenzyl)-2-hydroxyethyl]-9-octadecenamide (OMDM-2) or N-(4-hydroxy-2-methylphenyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (VDM-11; 10, 20 or 30 muM; each compound) delivered into the paraventricular thalamic nucleus (PVA) increased sleep and decreased waking. In addition, the infusion of compounds reduced the extracellular levels of dopamine collected from nucleus accumbens. Taken together, these findings illustrate a critical role of AMT in sleep modulation.
In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake.[Pubmed:14744610]
Eur J Pharmacol. 2004 Jan 26;484(2-3):249-57.
Two inhibitors of the cellular uptake of the endocannabinoid anandamide, (R)-N-oleoyl-(1'-hydroxybenzyl)-2'-ethanolamine and (S)-N-oleoyl-(1'-hydroxybenzyl)-2'-ethanolamine (OMDM-1 and OMDM-2, respectively), were recently synthesized, and their in vitro pharmacological activity described. Here we have assessed their activity in two typical pharmacological responses of cannabimimetic compounds. We first examined whether these compounds exert any effect per se on locomotion and pain perception in rats, and/or enhance the effects of anandamide on these two processes. We compared the effects of the novel compounds with those produced by a previously developed selective inhibitor, N-arachidonoyl-(2-methyl-4-hydroxyphenyl)amine (VDM-11). When assayed alone, OMDM-1 and OMDM-2 (1-10 mg/kg, i.p.) did not affect any of the five motor parameters under investigation, although the former compound exhibited a trend for the inhibition of ambulation, fast movements, and speed in rats. OMDM-2 and, to a lesser extent, VDM-11 (5 mg/kg, i.p.) enhanced the motor-inhibitory effects of a noneffective dose (2 mg/kg, i.p.) of anandamide, while OMDM-1 did not. In a typical test of acute analgesia, OMDM-2 and VDM-11 (1-10 mg/kg, i.p.), but not OMDM-1, significantly enhanced the time spent by rats on a "hot plate." However, the same compounds (5 mg/kg, i.p.) did not enhance the analgesic effect of a subeffective dose (2 mg/kg, i.p.) of anandamide, whereas OMDM-1 exerted a strong trend towards potentiation (P=0.06). We next explored the possible use of the two novel compounds in a pathological condition. Thus, we determined if, like other previously developed anandamide reuptake inhibitors, OMDM-1 and OMDM-2 inhibit spasticity in an animal model of multiple sclerosis-the chronic relapsing experimental allergic encephalomyelitis in mice. As previously shown with a higher dose of VDM-11, both novel compounds (5 mg/kg, i.v.) significantly reduced spasticity of the hindlimb in mice with chronic relapsing experimental allergic encephalomyelitis. We suggest that OMDM-1 and, particularly, OMDM-2 are useful pharmacological tools for the study of the (patho)physiological role of the anandamide cellular uptake process, and represent unique templates for the development of new antispastic drugs.
Novel selective and metabolically stable inhibitors of anandamide cellular uptake.[Pubmed:12732359]
Biochem Pharmacol. 2003 May 1;65(9):1473-81.
Novel aromatic analogues of N-oleoylethanolamine and N-arachidonoylethanolamine (anandamide, AEA) were synthesized and, based on the capability of similar compounds to interact with proteins of the endocannabinoid and endovanilloid signaling systems, were tested on: (i) cannabinoid CB(1) and CB(2) receptors; (ii) vanilloid VR1 receptors; (iii) anandamide cellular uptake (ACU); and (iv) the fatty acid amide hydrolase (FAAH). The (R)- and, particularly, the (S)-1'-(4-hydroxybenzyl) derivatives of N-oleoylethanolamine and AEA (OMDM-1, OMDM-2, OMDM-3, and OMDM-4) inhibited to a varied extent ACU in RBL-2H3 cells (K(i) ranging between 2.4 and 17.7 micro M), the oleoyl analogues (OMDM-1 and OMDM-2, K(i) 2.4 and 3.0 micro M, respectively) being 6- to 7-fold more potent than the arachidonoyl analogues (OMDM-3 and OMDM-4). These four compounds exhibited: (i) poor affinity for either CB(1) (K(i)> or = 5 micro M) or CB(2) (K(i)>10 micro M) receptors in rat brain and spleen membranes, respectively; (ii) almost no activity at vanilloid receptors in the intracellular calcium assay carried out with intact cells over-expressing the human VR1 (EC(50)> or = 10 micro M); and (iii) no activity as inhibitors of FAAH in N18TG2 cell membranes (K(i)>50 micro M). The oleoyl- and arachidonoyl-N'-(4-hydroxy-3-methoxybenzyl)hydrazines (OMDM-5 and OMDM-6), inhibited ACU (K(i) 4.8 and 7.0 micro M, respectively), and were more potent as VR1 agonists (EC(50) 75 and 50nM, respectively), weakly active as CB(1) receptor ligands (K(i) 4.9 and 3.2 micro M, respectively), and inactive as CB(2) ligands (K(i)>5 micro M) as well as on FAAH (K(i)> or = 40 micro M). In conclusion, we report two novel potent and selective inhibitors of ACU (OMDM-1 and OMDM-2) and one "hybrid" agonist of CB(1) and VR1 receptors (OMDM-6). Unlike other compounds of the same type, OMDM-1, OMDM-2, and OMDM-6 were very stable to enzymatic hydrolysis by rat brain homogenates.


