FuromolluginCAS# 61658-41-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61658-41-1 | SDF | Download SDF |
| PubChem ID | 10354359 | Appearance | Cryst. |
| Formula | C14H10O4 | M.Wt | 242.2 |
| Type of Compound | Naphthols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
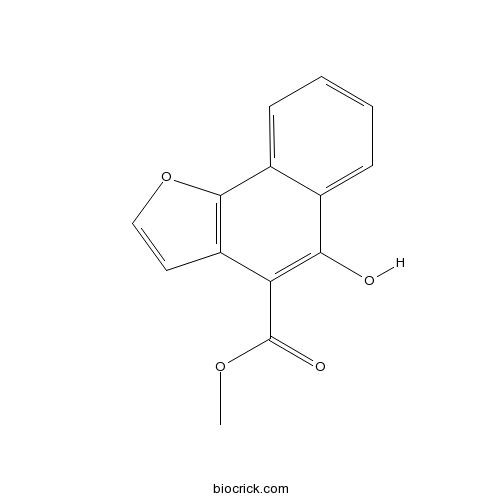
| Chemical Name | methyl 5-hydroxybenzo[g][1]benzofuran-4-carboxylate | ||
| SMILES | COC(=O)C1=C(C2=CC=CC=C2C3=C1C=CO3)O | ||
| Standard InChIKey | AFMYCYWCHKTNNE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O4/c1-17-14(16)11-10-6-7-18-13(10)9-5-3-2-4-8(9)12(11)15/h2-7,15H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Furomollugin has antiviral activity, it can strongly suppress the secretion of hepatitis B surface antigen (HBsAg), with IC50 = 2.0 micrograms/mL, in human hepatoma Hep3B cells. |
| Targets | Antifection |

Furomollugin Dilution Calculator

Furomollugin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1288 mL | 20.6441 mL | 41.2882 mL | 82.5764 mL | 103.2205 mL |
| 5 mM | 0.8258 mL | 4.1288 mL | 8.2576 mL | 16.5153 mL | 20.6441 mL |
| 10 mM | 0.4129 mL | 2.0644 mL | 4.1288 mL | 8.2576 mL | 10.322 mL |
| 50 mM | 0.0826 mL | 0.4129 mL | 0.8258 mL | 1.6515 mL | 2.0644 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4129 mL | 0.8258 mL | 1.0322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MK 212 hydrochloride
Catalog No.:BCC6856
CAS No.:61655-58-1
- Propargyl p-toluenesulfonate
Catalog No.:BCN2266
CAS No.:6165-76-0
- Propargyl benzenesulfonate
Catalog No.:BCN2247
CAS No.:6165-75-9
- Protopine hydrochloride
Catalog No.:BCN5345
CAS No.:6164-47-2
- Amfenac Sodium Monohydrate
Catalog No.:BCC4620
CAS No.:61618-27-7
- Songoroside A
Catalog No.:BCN3988
CAS No.:61617-29-6
- (-)-Sparteine Sulfate Pentahydrate
Catalog No.:BCC8273
CAS No.:6160-12-9
- Acetylcysteine
Catalog No.:BCC3716
CAS No.:616-91-1
- H-DL-Nle-OH
Catalog No.:BCC3301
CAS No.:616-06-8
- 13-Hydroxy-8,11,13-podocarpatrien-18-oic acid
Catalog No.:BCN1399
CAS No.:61597-83-9
- 15,16-Epoxy-12R-hydroxylabda-8(17),13(16),14-triene
Catalog No.:BCN1400
CAS No.:61597-55-5
- Vasicine
Catalog No.:BCN4140
CAS No.:6159-55-3
- 11-Methoxyuncarine C
Catalog No.:BCN4142
CAS No.:61665-08-5
- Neridienone B
Catalog No.:BCN4143
CAS No.:61671-56-5
- Trimethyl[3-(methylthio)propyl]ammonium(1+)
Catalog No.:BCN1398
CAS No.:61672-50-2
- 3-(Dimethylsulfonio)-N,N,N-trimethylpropanaminium(2+)
Catalog No.:BCN1397
CAS No.:61672-51-3
- H-Alaninol
Catalog No.:BCC2731
CAS No.:6168-72-5
- OMDM-2
Catalog No.:BCC5814
CAS No.:616884-63-0
- Ethyl vanillate
Catalog No.:BCN3670
CAS No.:617-05-0
- 2-Hydroxy-1-methoxyanthraquinone
Catalog No.:BCN3091
CAS No.:6170-06-5
- 2-Chloro-1,4-phenylenediamine sulfate
Catalog No.:BCN8435
CAS No.:61702-44-1
- W-5 hydrochloride
Catalog No.:BCC6621
CAS No.:61714-25-8
- W-7 hydrochloride
Catalog No.:BCC6622
CAS No.:61714-27-0
- Fluvoxamine maleate
Catalog No.:BCC1215
CAS No.:61718-82-9
Inhibition of hepatitis B surface antigen secretion on human hepatoma cells. Components from Rubia cordifolia.[Pubmed:8882438]
J Nat Prod. 1996 Mar;59(3):330-3.
The antiviral activity in the roots of Rubia cordifolia was examined, and three naphthohydroquinones, Furomollugin (1), mollugin (2), and rubilactone (3), were isolated from it. Compounds 1 and 2 strongly suppressed the secretion of hepatitis B surface antigen (HBsAg), both with IC50 = 2.0 micrograms/mL, in human hepatoma Hep3B cells while having little effect on the viability of the cells. Evaluation of structurally related derivatives of 1 and 2 revealed that a 6-hydroxy group and a pyran or furan ring contribute to this suppressive effect.
Phthalide Anions in Organic Synthesis. A Direct Total Synthesis of Furomollugin.[Pubmed:26197542]
Nat Prod Commun. 2015 Jun;10(6):1025-6.
Furomollugin was synthesized in three steps from commercially available starting materials using phthalide annulation chemistry.
A novel and efficient synthesis of diverse dihydronaphtho[1,2-b]furans using the ceric ammonium nitrate-catalyzed formal [3 + 2] cycloaddition of 1,4-naphthoquinones to olefins and its application to furomollugin.[Pubmed:23963248]
Org Biomol Chem. 2013 Sep 28;11(36):6097-107.
A novel approach was developed for the synthesis of diverse dihydronaphtho[1,2-b]furans from 1,4-naphthoquinones and olefins in the presence of ceric ammonium nitrate. This reaction provides a rapid route for the synthesis of a variety of dihydronaphtho[1,2-b]furans and naphtho[1,2-b]furans bearing different substituents. This methodology was also used to synthesize the biologically important natural product Furomollugin in only 2 steps.


