LumichromeCAS# 1086-80-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1086-80-2 | SDF | Download SDF |
| PubChem ID | 5326566 | Appearance | Powder |
| Formula | C12H10N4O2 | M.Wt | 242.23 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
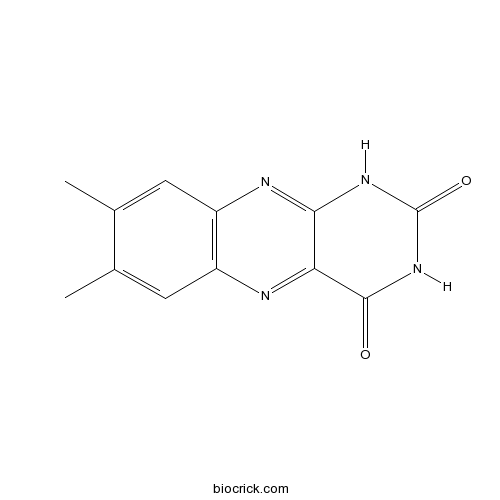
| Chemical Name | 7,8-dimethyl-1H-benzo[g]pteridine-2,4-dione | ||
| SMILES | CC1=CC2=C(C=C1C)N=C3C(=N2)C(=O)NC(=O)N3 | ||
| Standard InChIKey | ZJTJUVIJVLLGSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H10N4O2/c1-5-3-7-8(4-6(5)2)14-10-9(13-7)11(17)16-12(18)15-10/h3-4H,1-2H3,(H2,14,15,16,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lumichrome is a derivative of Riboflavin, a vitamin with a key role in maintaining cellular function and health in human and animals. 2. Lumichrome shows photosensitizing effects on the generation of volatiles in soy milk. |

Lumichrome Dilution Calculator

Lumichrome Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1283 mL | 20.6415 mL | 41.2831 mL | 82.5662 mL | 103.2077 mL |
| 5 mM | 0.8257 mL | 4.1283 mL | 8.2566 mL | 16.5132 mL | 20.6415 mL |
| 10 mM | 0.4128 mL | 2.0642 mL | 4.1283 mL | 8.2566 mL | 10.3208 mL |
| 50 mM | 0.0826 mL | 0.4128 mL | 0.8257 mL | 1.6513 mL | 2.0642 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4128 mL | 0.8257 mL | 1.0321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pyridostatin
Catalog No.:BCC1875
CAS No.:1085412-37-8
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- Ilexgenin A
Catalog No.:BCC9233
CAS No.:108524-94-3
- Ilexsaponin A
Catalog No.:BCN7867
CAS No.:108524-93-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- Fuligorubin A
Catalog No.:BCN1837
CAS No.:108343-55-1
- Noradrenaline bitartrate monohydrate
Catalog No.:BCC4810
CAS No.:108341-18-0
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
- Mizolastine
Catalog No.:BCC4521
CAS No.:108612-45-9
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
- Lupeol 3-hydroxyoctadecanoate
Catalog No.:BCN6686
CAS No.:108885-61-6
Optical spectroscopy of isolated flavins: photodissociation of protonated lumichrome.[Pubmed:29492507]
Phys Chem Chem Phys. 2018 Mar 14;20(11):7407-7414.
The optical properties of flavins strongly depend on the charge and oxidation states as well as the environment. Herein, the electronic spectrum of cold protonated Lumichrome, the smallest flavin molecule, is recorded by means of photodissociation in the visible range (VISPD) in a cryogenic ion trap tandem mass spectrometer coupled to an electrospray ionization source. The vibronic spectrum is assigned to the S1 <-- S0 (pipi*) transition of the most stable N5-protonated isomer by comparison with quantum chemical calculations at the PBE0/cc-pVDZ level in combination with multidimensional Franck-Condon simulations. Analysis of the geometric and electronic structures of neutral and protonated Lumichrome explains the large red shift of the band origin upon protonation (DeltaS1 approximately -6000 cm(-1)), which corresponds to the increase in proton affinity upon S1 excitation as a result of charge transfer. N5 protonation greatly modifies the structure of the central pyrazine ring of the chromophore. The orbitals involved in S1 <-- S0 excitation include an important fraction of the probability at the central ring and they are, hence, largely influenced by the positive charge of the attached proton. The rich vibronic spectrum indicates the large geometry change upon S1 excitation. This combined experimental and computational approach is shown to be suitable to determine the optical properties of flavins as a function of oxidation, protonation, metalation, and microsolvation state.
A New Dihydrochromone Dimer and Other Secondary Metabolites from Cultures of the Marine Sponge-Associated Fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213.[Pubmed:29194412]
Mar Drugs. 2017 Dec 1;15(12). pii: md15120375.
A previously unreported dihydrochromone dimer, paecilin E (1), was isolated, together with eleven known compounds: beta-sitostenone, ergosta-4,6,8 (14), 22-tetraen-3-one, cyathisterone, byssochlamic acid, dehydromevalonic acid lactone, chevalone B, aszonalenin, dankasterone A (2), helvolic acid, secalonic acid A and fellutanine A, from the culture filtrate extract of the marine sponge-associated fungus Neosartorya fennelliae KUFA 0811. Nine previously reported metabolites, including a chromanol derivative (3), (3beta, 5alpha, 22E), 3,5-dihydroxyergosta-7,22-dien-6-one (4), byssochlamic acid, hopan-3beta,22-diol, chevalone C, sartorypyrone B, helvolic acid, Lumichrome and the alkaloid harmane were isolated from the culture of the marine-sponge associated fungus Neosartorya tsunodae KUFC 9213. Paecilin E (1), dankasterone A (2), a chromanol derivative (3), (3beta, 5alpha, 22E)-3,5-dihydroxyergosta-7,22-dien-6-one (4), hopan-3beta,22-diol (5), Lumichrome (6), and harmane (7) were tested for their antibacterial activity against Gram-positive and Gram-negative reference and multidrug-resistant strains isolated from the environment. While paecilin E (1) was active against S. aureus ATCC 29213 and E. faecalis ATCC 29212, dankastetrone A (2) was only effective against E. faecalis ATCC 29212 and the multidrug-resistant VRE E. faecalis A5/102. Both compounds neither inhibit biofilm mass production in any of the strains at the concentrations tested nor exhibit synergistic association with antibiotics.
A freshwater diatom challenged by Zn: Biochemical, physiological and metabolomic responses of Tabellaria flocculosa(Roth) Kutzing.[Pubmed:29715753]
Environ Pollut. 2018 Jul;238:959-971.
Freshwater ecosystems are under threatening anthropogenic pressures worldwide, namely by metals. Diatoms are used as water quality indicators, but the influence of micronutrients such as Zn and its impacts are poorly understood. Thus, our study aimed to elucidate the tolerance level, the cellular targets and the responses to counteract Zn toxicity of freshwater diatoms by exposing Tabellaria flocculosa, isolated from a Zn contaminated stream. Biochemical, physiological and metabolomic approaches were used. It was demonstrated that Zn is toxic to T. flocculosa at concentrations occurring in contaminated environments. At low stress (30mug Zn/L) few alterations in the metabolome were observed, but the enzymatic (SOD, CAT) and molecular (GSH, GSSG) antioxidant systems were induced, protecting cells from oxidative stress. At moderate stress (500mug Zn/L) the main changes occurred in the metabolome (increases in fatty acids, amino acids, terpenoids, glycerol and phosphate, decreases in sucrose and Lumichrome) with a moderate increase in cell damage (LPO and PC). The concerted action of all these mechanisms resulted in a non-significant decrease of growth, explaining the survival of this T. flocculosa strain in an environment with this Zn concentration. At the highest stress level (1000mug Zn/L) the metabolome was identical to 500mug Zn/L, and the induction of antioxidant systems and extracellular ion chelation (exopolysaccharides, frustulins) were the main responses to the increase of Zn toxicity. However, these mechanisms were unable to effectively abrogate cellular damage and growth reduction was observed. Moreover, the decrease in sucrose and especially in Lumichrome should be tested as new specific markers of Zn toxicity. The information obtained in this study can assist in environmental risk assessment policies, support the prediction of diatom behaviour in highly impacted Zn environments, such as mining scenarios, and may help develop new indices, which include alterations induced by metals.
Fluorescent Molecular Probes for Detection of One-Electron Oxidants Photochemically Generated by Dissolved Organic Matter.[Pubmed:28723081]
Environ Sci Technol. 2017 Aug 15;51(16):9033-9041.
We report a dual probe system based on 4'-substituted biphenyl-2-carboxylic acids (BPAs) for analysis of photooxidants generated by dissolved organic matter. The BPA probes are converted to the corresponding benzocoumarins (BZCs) at different rates depending on the mechanism of oxidation; thus, two probes used simultaneously can differentiate strong triplet excited state sensitizers from hydroxylating species such as hydroxyl radical ((*)OH) present in dissolved organic matter (DOM). Comparison of the ratios of BZC-CH3 and BZC-CF3 product formation using model photooxidants such as NaNO2, a (*)OH precursor, and model triplet sensitizer Lumichrome gave a range of 2 to 250. Application of these probes to DOM isolates and whole natural waters afforded intermediate ratios. Although the oxidation potential of BPAs (>ca. 1.80 V SHE) is significantly higher than the estimated average reduction potential of typical (3)CDOM* samples, these results have demonstrated the presence of a small pool of oxidants in the selected DOM isolates and whole water samples that is capable of oxidizing aromatic carboxylates. As an analytical tool, this probe pair can be used between pH 4-6 without affecting the product formation ratio and may find applications in various systems involving complex mixtures of photochemically produced oxidants of differing natures.
Photo-induced proton-coupled electron transfer and dissociation of isolated flavin adenine dinucleotide mono-anions.[Pubmed:28745349]
Phys Chem Chem Phys. 2017 Oct 4;19(38):25829-25833.
The intrinsic optical absorption spectrum and photo-dissociation pathways of flavin adenine dinucleotide (FAD) mono-anions isolated in vacuo are probed using photo-induced dissociation (PID) action spectroscopy. The main photo-products are Lumichrome and formylmethylflavin. Evidence is presented that the dissociation pathway leading to these products is non-statistical i.e. occurs during the excited state lifetime. This suggests that the stacking of the adenine and alloxazine chromophores, which enables ultra-fast quenching of the flavin excited state by photo-induced electron transfer in aqueous solution, is inhibited in vacuo. These results provide firm experimental confirmation that Lumichrome formation from flavins proceeds via photo-induced, intra-molecular proton-coupled electron transfer.
Two-Component Flavin-Dependent Riboflavin Monooxygenase Degrades Riboflavin in Devosia riboflavina.[Pubmed:29610214]
J Bacteriol. 2018 May 24;200(12). pii: JB.00022-18.
The actinobacterium Microbacterium maritypicum splits riboflavin (vitamin B2) into Lumichrome and d-ribose. However, such degradation by other bacteria and the involvement of a two-component flavin-dependent monooxygenase (FMO) in the reaction remain unknown. Here we investigated the mechanism of riboflavin degradation by the riboflavin-assimilating alphaproteobacterium Devosia riboflavina (formerly Pseudomonas riboflavina). We found that adding riboflavin to bacterial cultures induced riboflavin-degrading activity and a protein of the FMO family that had 67% amino acid identity with the predicted riboflavin hydrolase (RcaE) of M. maritypicum MF109. The D. riboflavina genome clustered genes encoding the predicted FMO, flavin reductase (FR), ribokinase, and flavokinase, and riboflavin induced their expression. This finding suggests that these genes constitute a mechanism for utilizing riboflavin as a carbon source. Recombinant FMO (rFMO) protein of D. riboflavina oxidized riboflavin in the presence of reduced flavin mononucleotide (FMN) provided by recombinant FR (rFR), oxidized FMN and NADH, and produced stoichiometric amounts of Lumichrome and d-ribose. Further investigation of the enzymatic properties of D. riboflavina rFMO indicated that rFMO-rFR coupling accompanied O2 consumption and the generation of enzyme-bound hydroperoxy-FMN, which are characteristic of two-component FMOs. These results suggest that D. riboflavina FMO is involved in hydroperoxy-FMN-dependent mechanisms to oxygenize riboflavin and a riboflavin monooxygenase is necessary for the initial step of riboflavin degradation.IMPORTANCE Whether bacteria utilize either a monooxygenase or a hydrolase for riboflavin degradation has remained obscure. The present study found that a novel riboflavin monooxygenase, not riboflavin hydrolase, facilitated this process in D. riboflavina The riboflavin monooxygenase gene was clustered with flavin reductase, flavokinase, and ribokinase genes, and riboflavin induced their expression and riboflavin-degrading activity. The gene cluster is uniquely distributed in Devosia species and actinobacteria, which have exploited an environmental niche by developing adaptive mechanisms for riboflavin utilization.
[Studies on chemical constituents of Isodon excisoides].[Pubmed:28925118]
Zhongguo Zhong Yao Za Zhi. 2016 Sep;41(18):3361-3365.
The chemical constituents of the water extraction of the aerial parts of Isodon excisoides were investigated by various chromatographic methods including D-101 macroporous adsorptive resins, silica gel, Sephadex LH-20, MCI and semi-preparative HPLC. As a result, six compounds were separated and purified.By analyses of the HR-ESI-MS, 1D and 2D NMR spectra, their structures were determined as 3-O-beta-D-allopyranosyl-1-octen-3-ol(1), blumenolA (2), Lumichrome (3), loliolide(4), cirsiliol(5) and pedalitin(6). Compound 1 was a new compound, and compounds 2-4 were isolated from this plant for the first time.


