PSIProteasome inhibitor CAS# 158442-41-2 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-132
Catalog No.:BCC1227
CAS No.:133407-82-6
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

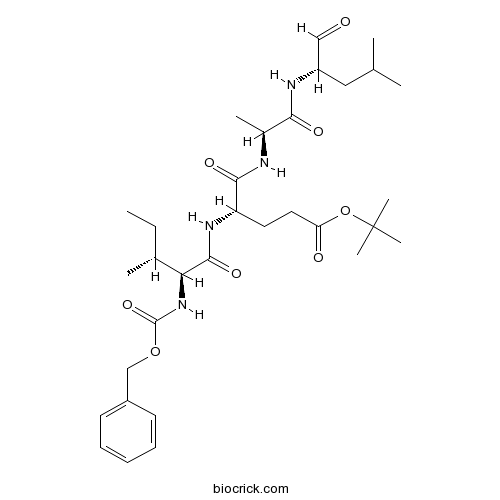
| Cas No. | 158442-41-2 | SDF | Download SDF |
| PubChem ID | 71464413 | Appearance | Powder |
| Formula | C32H50N4O8 | M.Wt | 618.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 30 mM in DMSO | ||
| Chemical Name | tert-butyl (4S)-5-[[(2S)-1-[[(2S)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-[[(2S,3R)-3-methyl-2-(phenylmethoxycarbonylamino)pentanoyl]amino]-5-oxopentanoate | ||
| SMILES | CCC(C)C(C(=O)NC(CCC(=O)OC(C)(C)C)C(=O)NC(C)C(=O)NC(CC(C)C)C=O)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | TYFTWYMXUWCOOB-XYXXBXNNSA-N | ||
| Standard InChI | InChI=1S/C32H50N4O8/c1-9-21(4)27(36-31(42)43-19-23-13-11-10-12-14-23)30(41)35-25(15-16-26(38)44-32(6,7)8)29(40)33-22(5)28(39)34-24(18-37)17-20(2)3/h10-14,18,20-22,24-25,27H,9,15-17,19H2,1-8H3,(H,33,40)(H,34,39)(H,35,41)(H,36,42)/t21-,22+,24+,25+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Proteasome inhibitor; inhibits chymotrypsin-like activity of the proteasome. Causes dopaminergic cell death in vitro. Prevents activation of NF-κB in response to TNF-α and okadaic acid by inhibiting IκB-α degradation. Also inhibits NO production by LPS-activated macrophages. |

PSI Dilution Calculator

PSI Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6161 mL | 8.0807 mL | 16.1614 mL | 32.3227 mL | 40.4034 mL |
| 5 mM | 0.3232 mL | 1.6161 mL | 3.2323 mL | 6.4645 mL | 8.0807 mL |
| 10 mM | 0.1616 mL | 0.8081 mL | 1.6161 mL | 3.2323 mL | 4.0403 mL |
| 50 mM | 0.0323 mL | 0.1616 mL | 0.3232 mL | 0.6465 mL | 0.8081 mL |
| 100 mM | 0.0162 mL | 0.0808 mL | 0.1616 mL | 0.3232 mL | 0.404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Light fluctuation induced photoinhibiton of PSI can be alleviated by enhancing CEF-PSI.
Abstract
StAsp-PSI time-dependently destabilizes the bilayers of cell membrane, interacts with phospholipids and aggregates to form pores within cell membrane.
Abstract
Psi is an indispensible component involved in the packaging of gRNA into HIV-1 particles, where Gag NC domain binds to Psi with a reduced Kd(1M) and relatively low Zeff. However the nonelectrostatic component of binding is significantly reduced if any changes occur in the NC zinc finger of Gag or in the G-rich NC-binding regions of Psi RNA.
Abstract
PSI is a polysaccharide that is stochastically expressd by C. difficile. The synthesized PSI pentasaccharide is used to produce a potential dual C. difficile vaccine and can be detected by natural anti-PSI IgG antibodies if unphosphorylated.
Abstract
Protein biological functions are characterized based on protein structures in PSI:Biology, which is the 3rd phase of PSI and can be achieved by participation of numerous research institutions.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ZIE(OtBu)AL-CHO (PSI)1 have been shown to inhibit the proteasome activities in a variety of cell types.
Peptide aldehyde, PSI (Z-Ile-Glu(OtBu)-Ala-Leu-al), inhibits the proteasome 10-fold better than calpain but is less potent than MG1322. Since MG132, PSI, MG115 (Z-Leu-Leu-nVal-al) and ALLN can all inhibit calpains and various lysosomal cathepsins in addition to the proteasome, when using these inhibitors in cell culture it is important to perform control experiments to con¢rm that the observed e¡ects are due to the inhibition of the proteasome. First, one can use agents, which block intracellular cysteine proteases, but do not inhibit proteasomes3. Such inhibitors are Z-Leu-Leu- al, and E-64 for calpains4, and weak bases such as chloroquine and E-64 for lysosomal proteolysis . In yeast, where digestive vacuoles contain mainly serine, not cysteine, proteases, phenylmethylsulfonyl £uoride can be used to inhibit these enzymes without affecting proteasomes5.
Despite the availability of these inhibitors, MG132, due to its low cost and the rapid reversibility of its action, still remains, in our opinion, the first choice to study proteasome involvement in a process in cell cultures or tissues, if appropriate controls are used. As the most potent and selective of commercially available aldehydes, MG132 is preferable to ALLN, MG115 (Z-Leu-Leu-nVal-al), or even PSI. On the other hand, the least selective inhibitor, ALLN, because of its ability to inhibit most major pro teases in mammalian cells, is probably the best tool for prevention of unwanted proteolysis, for example during isolation of proteins from mammalian cells.
Reference:
1. Takada K (1995) Mol. Biol. Rep. 21: 21–26
2. A. F. Kisselev, A. L. Goldberg. Proteasome inhibitors: from research tools to drug candidates. Chemistry & Biology 8 (2001) 739-758.
3. W. Matthews, J. Driscoll, K. Tanaka, A. Ichihara, A.L. Goldberg, Involvement of the proteasome in various degradative processes in mammalian cells, Proc. Natl. Acad. Sci. USA 86 (1989) 2597-2601.
4. S. Tsubuki, Y. Saito, M. Tomioka, H. Ito, S. Kawashima, Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine, J. Biochem. 119 (1996) 572-576.
5. D.H. Lee, A.L. Goldberg, Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae, J. Biol. Chem. 271 (1996) 27280-27284.
- Demethyl calyciphylline A
Catalog No.:BCN7040
CAS No.:1584236-34-9
- Pyraclonil
Catalog No.:BCC8073
CAS No.:158353-15-2
- Methyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4051
CAS No.:15823-04-8
- Naringenin-7-O-glucuronide
Catalog No.:BCC8215
CAS No.:158196-34-0
- Fmoc-Ser(HPO3Bzl)-OH
Catalog No.:BCC3543
CAS No.:158171-14-3
- 21-O-Tigloylgymnemagenin
Catalog No.:BCN7854
CAS No.:1581276-63-2
- 5-Hydroxy-6,7,8-trimethoxycoumarin
Catalog No.:BCN7470
CAS No.:1581248-32-9
- para-iodoHoechst 33258
Catalog No.:BCC1838
CAS No.:158013-43-5
- meta-iodoHoechst 33258
Catalog No.:BCC1739
CAS No.:158013-42-4
- ortho-iodoHoechst 33258
Catalog No.:BCC1824
CAS No.:158013-41-3
- Chitinase-IN-2
Catalog No.:BCC5534
CAS No.:1579991-63-1
- Chitinase-IN-1
Catalog No.:BCC5533
CAS No.:1579991-61-9
- BMS-983970
Catalog No.:BCC5509
CAS No.:1584713-87-0
- ω-Agatoxin TK
Catalog No.:BCC7489
CAS No.:158484-42-5
- Catalpalactone
Catalog No.:BCN1708
CAS No.:1585-68-8
- Cnidioside B methyl ester
Catalog No.:BCN1707
CAS No.:158500-59-5
- Eclalbasaponin I
Catalog No.:BCN8244
CAS No.:158511-59-2
- Ombuin 3-glucoside
Catalog No.:BCN4055
CAS No.:158642-42-3
- Rimonabant hydrochloride
Catalog No.:BCC1898
CAS No.:158681-13-1
- Dihydrexidine hydrochloride
Catalog No.:BCC5681
CAS No.:158704-02-0
- 2-Naphthyl N-benzoylphenylalaninate
Catalog No.:BCC8583
CAS No.:15873-25-3
- Aescin IIA
Catalog No.:BCN6551
CAS No.:158732-55-9
- Salvianolic acid F
Catalog No.:BCN2924
CAS No.:158732-59-3
- Escin IIB
Catalog No.:BCN8127
CAS No.:158800-83-0
De novo [PSI (+)] prion formation involves multiple pathways to form infectious oligomers.[Pubmed:28250435]
Sci Rep. 2017 Mar 6;7(1):76.
Prion and other neurodegenerative diseases are associated with misfolded protein assemblies called amyloid. Research has begun to uncover common mechanisms underlying transmission of amyloids, yet how amyloids form in vivo is still unclear. Here, we take advantage of the yeast prion, [PSI (+)], to uncover the early steps of amyloid formation in vivo. [PSI (+)] is the prion form of the Sup35 protein. While [PSI (+)] formation is quite rare, the prion can be greatly induced by overexpression of the prion domain of the Sup35 protein. This de novo induction of [PSI (+)] shows the appearance of fluorescent cytoplasmic rings when the prion domain is fused with GFP. Our current work shows that de novo induction is more complex than previously thought. Using 4D live cell imaging, we observed that fluorescent structures are formed by four different pathways to yield [PSI (+)] cells. Biochemical analysis of de novo induced cultures indicates that newly formed SDS resistant oligomers change in size over time and lysates made from de novo induced cultures are able to convert [PSI (-)] cells to [PSI (+)] cells. Taken together, our findings suggest that newly formed prion oligomers are infectious.
Stromal Loop of Lhca6 is Responsible for the Linker Function Required for the NDH-PSI Supercomplex Formation.[Pubmed:28184910]
Plant Cell Physiol. 2017 Apr 1;58(4):851-861.
The light-harvesting complex I (LHCI) proteins in ArabidoPSIs thaliana are encoded by six genes. Major LHCI proteins (Lhca1-Lhca4) harvest light energy and transfer the resulting excitation energy to the PSI core by forming a PSI supercomplex. In contrast, the minor LHCI proteins Lhca5 and Lhca6 contribute to supercomplex formation between the PSI supercomplex and the chloroplast NADH dehydrogenase-like (NDH) complex, although Lhca5 is also solely associated with the PSI supercomplex. Lhca6 was branched from Lhca2 during the evolution of land plants. In this study, we focused on the molecular evolution involved in the transition from a major LHCI, Lhca2, to the linker protein Lhca6. To elucidate the domains of Lhca6 responsible for linker function, we systematically swapped domains between the two LHCI proteins. To overcome problems due to the low stability of chimeric proteins, we employed sensitive methods to evaluate supercomplex formation: we monitored NDH activity by using Chl fluorescence analysis and detected NDH-PSI supercomplex formation by using protein blot analysis in the form of two-dimensional blue-native (BN)/SDS-PAGE. The stromal loop of Lhca6 was shown to be necessary and sufficient for linker function. Chimeric Lhca6, in which the stromal loop was substituted by that of Lhca2, was not functional as a linker and was detected at the position of the PSI supercomplex in the BN-polyacrylamide gel. The stromal loop of Lhca6 is likely to be necessary for the interaction with chloroplast NDH, rather than for the association with the PSI supercomplex.
Precise Measurement of the e^{+}e^{-}-->pi^{+}pi^{-}J/psi Cross Section at Center-of-Mass Energies from 3.77 to 4.60 GeV.[Pubmed:28306266]
Phys Rev Lett. 2017 Mar 3;118(9):092001.
The cross section for the process e^{+}e^{-}-->pi^{+}pi^{-}J/PSI is measured precisely at center-of-mass energies from 3.77 to 4.60 GeV using 9 fb^{-1} of data collected with the BESIII detector operating at the BEPCII storage ring. Two resonant structures are observed in a fit to the cross section. The first resonance has a mass of (4222.0+/-3.1+/-1.4) MeV/c^{2} and a width of (44.1+/-4.3+/-2.0) MeV, while the second one has a mass of (4320.0+/-10.4+/-7.0) MeV/c^{2} and a width of (101.4_{-19.7}^{+25.3}+/-10.2) MeV, where the first errors are statistical and second ones are systematic. The first resonance agrees with the Y(4260) resonance reported by previous experiments. The precision of its resonant parameters is improved significantly. The second resonance is observed in e^{+}e^{-}-->pi^{+}pi^{-}J/PSI for the first time. The statistical significance of this resonance is estimated to be larger than 7.6sigma. The mass and width of the second resonance agree with the Y(4360) resonance reported by the BABAR and Belle experiments within errors. Finally, the Y(4008) resonance previously observed by the Belle experiment is not confirmed in the description of the BESIII data.
Disrupting the cortical actin cytoskeleton points to two distinct mechanisms of yeast [PSI+] prion formation.[Pubmed:28369054]
PLoS Genet. 2017 Apr 3;13(4):e1006708.
Mammalian and fungal prions arise de novo; however, the mechanism is poorly understood in molecular terms. One strong possibility is that oxidative damage to the non-prion form of a protein may be an important trigger influencing the formation of its heritable prion conformation. We have examined the oxidative stress-induced formation of the yeast [PSI+] prion, which is the altered conformation of the Sup35 translation termination factor. We used tandem affinity purification (TAP) and mass spectrometry to identify the proteins which associate with Sup35 in a tsa1 tsa2 antioxidant mutant to address the mechanism by which Sup35 forms the [PSI+] prion during oxidative stress conditions. This analysis identified several components of the cortical actin cytoskeleton including the Abp1 actin nucleation promoting factor, and we show that deletion of the ABP1 gene abrogates oxidant-induced [PSI+] prion formation. The frequency of spontaneous [PSI+] prion formation can be increased by overexpression of Sup35 since the excess Sup35 increases the probability of forming prion seeds. In contrast to oxidant-induced [PSI+] prion formation, overexpression-induced [PSI+] prion formation was only modestly affected in an abp1 mutant. Furthermore, treating yeast cells with latrunculin A to disrupt the formation of actin cables and patches abrogated oxidant-induced, but not overexpression-induced [PSI+] prion formation, suggesting a mechanistic difference in prion formation. [PIN+], the prion form of Rnq1, localizes to the IPOD (insoluble protein deposit) and is thought to influence the aggregation of other proteins. We show Sup35 becomes oxidized and aggregates during oxidative stress conditions, but does not co-localize with Rnq1 in an abp1 mutant which may account for the reduced frequency of [PSI+] prion formation.
An immunohistochemical and stereological analysis of PSI-induced nigral neuronal degeneration in the rat.[Pubmed:19187437]
J Neurochem. 2009 Apr;109(1):52-9.
Systemic administration of the proteasomal inhibitor I (PSI) to rats was reported to cause progressive nigral dopaminergic neuronal loss but this is disputed. A major controversy centres over the use of manual counting of tyrosine hydroxylase (TH) positive neurons at the level of third cranial nerve as opposed to employing systematic stereological analysis of cell loss in the entire substantia nigra (SN). To provide a method of marking SN neurones independent of protein expression, fluorogold (FG) was stereotaxically injected bilaterally into the striatum of male Wistar rats to retrogradely label nigral dopaminergic neurons. After 1 week, animals were treated with six doses of PSI (8 mg/kg, s.c.) or its vehicle (dimethyl sulphoxide) on alternate days over a 2-week period. Five weeks after the last treatment, PSI-treated animals showed decreased spontaneous locomotor activity and reduced TH positive SN cell number at the level of the third cranial nerve compared to control rats. Manual cell counting showed loss of FG-labelled SN neurones at this level, with a subpopulation of surviving neurons displaying abnormal morphology. Manual counting of all FG-labelled cells in the entire SN also showed regional PSI-induced loss of neurones with both normal and compromised morphology. Stereological optical fractionator estimates of total FG-labelled cell number confirmed the manual cell counting data both at the level of the third cranial nerve and throughout the entire SN. These findings confirm that PSI does cause a persistent nigral dopaminergic neuronal loss. The reason for the lack of reproducibility between laboratories requires further investigation. We suggest that a failure to distinguish between TH-positive neurones with normal and abnormal morphology following PSI administration contributes to equivocal results.
A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B.[Pubmed:7957109]
EMBO J. 1994 Nov 15;13(22):5433-41.
Activation of the inducible transcription factor NF-kappa B involves removal of the inhibitory subunit I kappa B-alpha from a latent cytoplasmic complex. It has been reported that I kappa B-alpha is subject to both phosphorylation and proteolysis in the process of NF-kappa B activation. In this study, we present evidence that the multicatalytic cytosolic protease (proteasome) is involved in the degradation of I kappa B-alpha. Micromolar amounts of the peptide Cbz-Ile-Glu(O-t-Bu)-Ala-leucinal (PSI), a specific inhibitor of the chymotryPSIn-like activity of the proteasome, prevented activation of NF-kappa B in response to tumor necrosis factor-alpha (TNF) and okadaic acid (OA) through inhibition of I kappa B-alpha degradation. The m-calpain inhibitor Cbz-Leu-leucinal was ineffective. In the presence of PSI, a newly phosphorylated form of I kappa B-alpha accumulated in TNF- and OA-stimulated cells. However, the covalent modification of I kappa B-alpha was not sufficient for activation of NF-kappa B: no substantial NF-kappa B DNA binding activity appeared in cells because the newly phosphorylated form of I kappa B-alpha was still tightly bound to p65 NF-kappa B. Pyrrolidinedithiocarbamate, an antioxidant inhibitor of NF-kappa B activation which did not interfere with proteasome activities, prevented de novo phosphorylation of I kappa B-alpha as well as its subsequent degradation. This suggests that phosphorylation of I kappa B-alpha is equally necessary for the activation of NF-kappa B.(ABSTRACT TRUNCATED AT 250 WORDS)


