ortho-iodoHoechst 33258Blue fluorescent dyes CAS# 158013-41-3 |
- TAK-700 salt
Catalog No.:BCC1979
CAS No.:426219-53-6
- TAK-700 R-form
Catalog No.:BCC4203
CAS No.:752243-39-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 158013-41-3 | SDF | Download SDF |
| PubChem ID | 10324821 | Appearance | Powder |
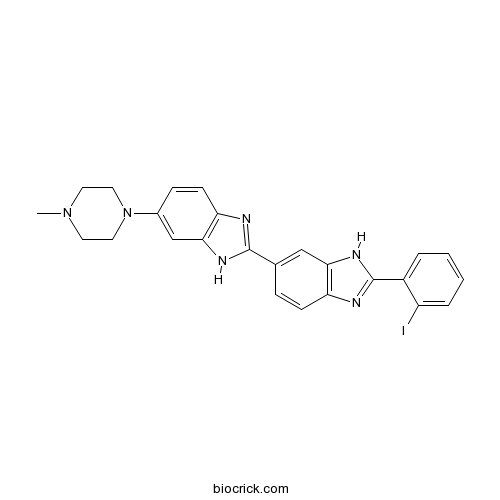
| Formula | C25H23IN6 | M.Wt | 534.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO or water Protect from light | ||
| Chemical Name | 2-(2-iodophenyl)-6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazole | ||
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)N=C(N5)C6=CC=CC=C6I | ||
| Standard InChIKey | UOHARKYRPCGBEI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H23IN6/c1-31-10-12-32(13-11-31)17-7-9-21-23(15-17)29-24(27-21)16-6-8-20-22(14-16)30-25(28-20)18-4-2-3-5-19(18)26/h2-9,14-15H,10-13H2,1H3,(H,27,29)(H,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

ortho-iodoHoechst 33258 Dilution Calculator

ortho-iodoHoechst 33258 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8713 mL | 9.3565 mL | 18.7129 mL | 37.4259 mL | 46.7823 mL |
| 5 mM | 0.3743 mL | 1.8713 mL | 3.7426 mL | 7.4852 mL | 9.3565 mL |
| 10 mM | 0.1871 mL | 0.9356 mL | 1.8713 mL | 3.7426 mL | 4.6782 mL |
| 50 mM | 0.0374 mL | 0.1871 mL | 0.3743 mL | 0.7485 mL | 0.9356 mL |
| 100 mM | 0.0187 mL | 0.0936 mL | 0.1871 mL | 0.3743 mL | 0.4678 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- Chitinase-IN-2
Catalog No.:BCC5534
CAS No.:1579991-63-1
- Chitinase-IN-1
Catalog No.:BCC5533
CAS No.:1579991-61-9
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- 5-Benzimidazolecarboxylic acid
Catalog No.:BCC8739
CAS No.:15788-16-6
- 5-Nonyloxytryptamine oxalate
Catalog No.:BCC6839
CAS No.:157798-13-5
- Gypenoside A
Catalog No.:BCN8459
CAS No.:157752-01-7
- Gentiournoside D
Catalog No.:BCN7855
CAS No.:157722-21-9
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- 7-Xylosyl-10-deacetylbaccatin III
Catalog No.:BCN7668
CAS No.:157664-03-4
- Prenylpiperitol
Catalog No.:BCN1706
CAS No.:157659-20-6
- NSC 632839 hydrochloride
Catalog No.:BCC2088
CAS No.:157654-67-6
- Boc-Pro-OH
Catalog No.:BCC3435
CAS No.:15761-39-4
- meta-iodoHoechst 33258
Catalog No.:BCC1739
CAS No.:158013-42-4
- para-iodoHoechst 33258
Catalog No.:BCC1838
CAS No.:158013-43-5
- 5-Hydroxy-6,7,8-trimethoxycoumarin
Catalog No.:BCN7470
CAS No.:1581248-32-9
- 21-O-Tigloylgymnemagenin
Catalog No.:BCN7854
CAS No.:1581276-63-2
- Fmoc-Ser(HPO3Bzl)-OH
Catalog No.:BCC3543
CAS No.:158171-14-3
- Naringenin-7-O-glucuronide
Catalog No.:BCC8215
CAS No.:158196-34-0
- Methyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4051
CAS No.:15823-04-8
- Pyraclonil
Catalog No.:BCC8073
CAS No.:158353-15-2
- Demethyl calyciphylline A
Catalog No.:BCN7040
CAS No.:1584236-34-9
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- BMS-983970
Catalog No.:BCC5509
CAS No.:1584713-87-0
- ω-Agatoxin TK
Catalog No.:BCC7489
CAS No.:158484-42-5
Strong positive cooperativity in binding to the A3T3 repeat by Hoechst 33258 derivatives attaching the quinoline units at the end of a branched linker.[Pubmed:26154241]
Bioorg Med Chem. 2015 Aug 1;23(15):4583-90.
Hoechst 33258 derivatives with additional interacting moieties attached at the ends of branched linkers were synthesized, and their DNA binding properties were investigated with regard to the A3T3 repeat by measuring fluorescence spectra. The binding property of the ligand was investigated by fluorescence titration, and the titration data were analyzed using the McGhee-von Hippel method. Ligand 6Q with the quinolin-6-yloxyacetyl group and Ligand IQ with isoquinolin-6-yloxyacetyl group at the ends of the branched linkers exhibit highly positive cooperativity for the DNA having 5 A3T3 sites with 3 base-insertions between them with sequence selectivity. The strategy developed in this study may be generally applicable for designing ligands for repetitive DNA sequences.
Aggregation features and fluorescence of Hoechst 33258.[Pubmed:25759973]
J Phys Chem B. 2015 Apr 2;119(13):4575-81.
The functionality of the bisbenzimide Hoechst 33258 in solution has been largely exploited in the quantification of DNA. Understanding of its behavior is essential to promote its widespread application and learning of biological processes. A detailed study of the dimerization process of the fluorescent blue dye Hoechst 33258 is carried out by isothermal titration calorimetry, absorbance, fluorescence, differential scanning calorimetry and T-jump kinetic measurements. The dimer/monomer ratio depends on the dye concentration and the ionic strength. The dimerization constant determined under physiological conditions (pH = 7.0; I = 0.10 M), KD = 3 x 10(4) M(-1), conveys that only micromolar concentrations of the dye can ensure reasonably high amounts of the monomer species in solution. For instance, for 10 muM dye content, the dimer prevails for I > 0.08 M, whereas the monomer is observed at low ionic strength, a key issue to be elucidated as long as the dimer species is more fluorescent than the monomer and the fluorescence intensity strongly relies on the ionic strength and the dye concentration.
Highly Sensitive Fluorescence Quantitative Detection of Mercury in Soil Based on Non-labeled Molecular Beacon and Fluorescent Dye Hoechst 33258.[Pubmed:28302966]
Anal Sci. 2017;33(3):275-279.
A highly sensitive and selective fluorescence method of quantitative detection for mercury in soil was developed using non-labeled molecular beacon (MB), single-stranded nucleic acid (ssDNA) and fluorescent dye Hoechst 33258. In this analytical method, the loop of MB was designed to be a sequence that was complementary to the ssDNA with multiple T-T mismatches, the stem of MB was completely designed as C-G base pairs, and both ends of the MB are not modified by any fluorophore and quencher. In the absence of Hg(2+), the interaction between Hoechst 33258 and the MB was very weak, and the fluorescence signal of Hoechst 33258 was very low. In the presence of Hg(2+), the MB and ssDNA with multiple T-T mismatches formed a double-stranded nucleic acid (dsDNA) via the T-Hg(2+)-T coordination structure which provided binding sites for Hoechst 33258. Then Hoechst 33258 binded to A-T base pairs of dsDNA, and the fluorescence intensity of Hoechst 33258 was significantly enhanced. Thus, a highly sensitive fluorescence quantitative detection method for Hg(2+) can be realized. In this strategy, the optimal determination conditions for Hg(2+) were a buffer solution pH of 8.2, an incubated temperature of 50 degrees C, an incubated time of 5 min and NaCl of 60 mmol L(-1). Under the optimum conditions, the fluorescence intensity of Hoechst 33258 exhibited a good linear dependence on the concentration of Hg(2+) in the range of 5 x 10(-9) - 400 x 10(-9) mol L(-1). The fitted regression equation was DeltaI = 2.1084C - 8.9587 with a correlation coefficient of 0.9943 (R(2)), and the detection limit of this method was 3 x 10(-9) mol L(-1) (3sigma). The proposed method had a high selection; the common substances in soil such as Ca(2+), Mg(2+), Mn(2+), Fe(3+), Cu(2+), Pb(2+), Al(3+), K(+), Na(+), Ni(2+), Cd(2+), Cr(3+), SiO3(2-), Cl(-), PO4(3-), NH4(+) and S(2-) had no interference to the detection of mercury. The proposed method had a high accuracy, and it was applied to detect mercury of ten different types of soil; the recoveries were 97.65 - 103.22%. In addition, the proposed method had a low background emission, fast detection speed and low detection cost.
UV-induced spectral shift and protonation of DNA fluorescent dye Hoechst 33258.[Pubmed:25312832]
J Fluoresc. 2014 Nov;24(6):1791-801.
DNA-bound Hoechst 33258 is readily excited with UV light and emits blue fluorescence, however, upon exposure to UV, the dye undergoes photobleaching as well as photoconversion to a blue-excited green-emitting form. We demonstrate that the UV-generated green-emitting form of Hoechst 33258 exhibits spectral properties very similar to the form of the dye that can be obtained by subjecting it to an acidic environment (pH 0.5-3.0). We also demonstrate that exposure of Hoechst 33258 to UV light (or hydrogen peroxide) leads to generation of the protonated (1+, 2+, 3+ and possibly the 4+) forms of the dye. Photoconversion of Hoechst 33258 has recently been exploited in single molecule localisation microscopy, thus understanding photophysics of this process can facilitate further development of high resolution optical imaging.


