Naringenin-7-O-glucuronideCAS# 158196-34-0 |
Quality Control & MSDS
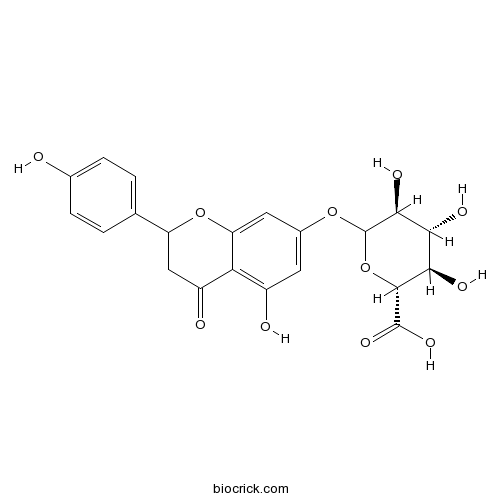
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 158196-34-0 | SDF | Download SDF |
| PubChem ID | 133612151 | Appearance | Powder |
| Formula | C21H20O11 | M.Wt | 448.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Naringenin 7-O-beta-D-glucuronide | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4R,5S)-3,4,5-trihydroxy-6-[[5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-2,3-dihydrochromen-7-yl]oxy]oxane-2-carboxylic acid | ||
| SMILES | C1C(OC2=CC(=CC(=C2C1=O)O)OC3C(C(C(C(O3)C(=O)O)O)O)O)C4=CC=C(C=C4)O | ||
| Standard InChIKey | BDCRTIDKZGEVEN-IDPBCYSYSA-N | ||
| Standard InChI | InChI=1S/C21H20O11/c22-9-3-1-8(2-4-9)13-7-12(24)15-11(23)5-10(6-14(15)31-13)30-21-18(27)16(25)17(26)19(32-21)20(28)29/h1-6,13,16-19,21-23,25-27H,7H2,(H,28,29)/t13?,16-,17-,18+,19-,21?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Naringenin-7-O-glucuronide Dilution Calculator

Naringenin-7-O-glucuronide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2302 mL | 11.1508 mL | 22.3015 mL | 44.603 mL | 55.7538 mL |
| 5 mM | 0.446 mL | 2.2302 mL | 4.4603 mL | 8.9206 mL | 11.1508 mL |
| 10 mM | 0.223 mL | 1.1151 mL | 2.2302 mL | 4.4603 mL | 5.5754 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.446 mL | 0.8921 mL | 1.1151 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.446 mL | 0.5575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Ser(HPO3Bzl)-OH
Catalog No.:BCC3543
CAS No.:158171-14-3
- 21-O-Tigloylgymnemagenin
Catalog No.:BCN7854
CAS No.:1581276-63-2
- 5-Hydroxy-6,7,8-trimethoxycoumarin
Catalog No.:BCN7470
CAS No.:1581248-32-9
- para-iodoHoechst 33258
Catalog No.:BCC1838
CAS No.:158013-43-5
- meta-iodoHoechst 33258
Catalog No.:BCC1739
CAS No.:158013-42-4
- ortho-iodoHoechst 33258
Catalog No.:BCC1824
CAS No.:158013-41-3
- Chitinase-IN-2
Catalog No.:BCC5534
CAS No.:1579991-63-1
- Chitinase-IN-1
Catalog No.:BCC5533
CAS No.:1579991-61-9
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- 5-Benzimidazolecarboxylic acid
Catalog No.:BCC8739
CAS No.:15788-16-6
- 5-Nonyloxytryptamine oxalate
Catalog No.:BCC6839
CAS No.:157798-13-5
- Gypenoside A
Catalog No.:BCN8459
CAS No.:157752-01-7
- Methyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4051
CAS No.:15823-04-8
- Pyraclonil
Catalog No.:BCC8073
CAS No.:158353-15-2
- Demethyl calyciphylline A
Catalog No.:BCN7040
CAS No.:1584236-34-9
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- BMS-983970
Catalog No.:BCC5509
CAS No.:1584713-87-0
- ω-Agatoxin TK
Catalog No.:BCC7489
CAS No.:158484-42-5
- Catalpalactone
Catalog No.:BCN1708
CAS No.:1585-68-8
- Cnidioside B methyl ester
Catalog No.:BCN1707
CAS No.:158500-59-5
- Eclalbasaponin I
Catalog No.:BCN8244
CAS No.:158511-59-2
- Ombuin 3-glucoside
Catalog No.:BCN4055
CAS No.:158642-42-3
- Rimonabant hydrochloride
Catalog No.:BCC1898
CAS No.:158681-13-1
- Dihydrexidine hydrochloride
Catalog No.:BCC5681
CAS No.:158704-02-0
Exploring the C-Terminal Tail Dynamics: Structural and Molecular Perspectives into the Therapeutic Activities of Novel CRMP-2 Inhibitors, Naringenin and Naringenin-7-O-glucuronide, in the Treatment of Alzheimer's Disease.[Pubmed:30290062]
Chem Biodivers. 2018 Dec;15(12):e1800437.
The collapsin response mediator protein (CRMP-2) is hyperphosphorylated in Alzheimer's disease (AD). These phosphorylation events are mediated by specific kinase proteins, GSK3beta and Cdk5, and occur at target phosphorylation sites majorly located at the C-terminal tail of CRMP-2. The abilities of naringenin (NAR) and Naringenin-7-O-glucuronide (NAR-7-O-G) to selectively bind CRMP-2 and reduce its phosphorylation have been previously demonstrated; the molecular interplay between these events remains unresolved. Using computational tools, we unravel the possible mechanisms by which these molecules disrupt CRMP-2 phosphorylation. Structural and dynamic analyses revealed that while the C-terminal tail of unbound CRMP-2 was extended and subtly organized, notable conformational disarray and rigidity characterized this region when bound by NAR and NAR-7-O-G. Consequentially, atomistic motions of constituent phosphorylation sites were restricted, indicative of structural occurrences that could distort the accessibility of interactive kinase proteins. A similar pattern was observed at a target phosphorylation site located in the globular domain of CRMP-2. MM/PBSA analyses revealed that both compounds interacted favorably with CRMP-2 while crucial residues that enhanced their selective binding include Glu353, Thr349, Lys254, Asp140 and Arg75. These structural insights provide mechanistic events that could contribute towards the structure-based design of anti-AD molecules which can bind CRMP2 selectively and alter its phosphorylation process.
Bioavailability of Bergamot (Citrus bergamia) Flavanones and Biological Activity of Their Circulating Metabolites in Human Pro-Angiogenic Cells.[Pubmed:29211032]
Nutrients. 2017 Dec 6;9(12). pii: nu9121328.
Myeloid angiogenic cells (MACs) play a key role in endothelial repairing processes and functionality but their activity may be impaired by the lipotoxic effects of some molecules like stearic acid (SA). Among the dietary components potentially able to modulate endothelial function in vivo, (poly)phenolic compounds represent serious candidates. Here, we apply a comprehensive multidisciplinary approach to shed light on the prospects of Bergamot (Citrus bergamia), a citrus fruit rich in flavanones and other phenolic compounds, in the framework of lipotoxicity-induced MACs impairment. The flavanone profile of bergamot juice was characterized and 16 compounds were identified, with a new 3-hydroxy-3-methylglutaryl (HMG) flavanone, isosakuranetin-7-O-neohesperidoside-6''-O-HMG, described for the first time. Then, a pilot bioavailability study was conducted in healthy volunteers to assess the circulating flavanone metabolites in plasma and urine after consumption of bergamot juice. Up to 12 flavanone phase II conjugates (sulfates and glucuronides of hesperetin, naringenin and eriodyctiol) were detected and quantified. Finally, the effect of some of the metabolites identified in vivo, namely hesperetin-7-O-glucuronide, hesperetin-3'-O-glucuronide, Naringenin-7-O-glucuronide and naringenin-4'-O-glucuronide, was tested, at physiological concentrations, on gene expression of inflammatory markers and apoptosis in MACs exposed to SA. Under these conditions, naringenin-4'-O-glucuronide and hesperetin-7-O-glucuronide were able to modulate inflammation, while no flavanone glucuronide was effective in curbing stearate-induced lipoapoptosis. These results demonstrate that some flavanone metabolites, derived from the in vivo transformation of bergamot juice phenolics in humans, may mitigate stearate-induced inflammation in MACs.
Effects of naringenin and its phase II metabolites on in vitro human macrophage gene expression.[Pubmed:23883170]
Int J Food Sci Nutr. 2013 Nov;64(7):843-9.
Naringenin, together with its glycosidic forms, is a flavanone abundant in grapefruit and orange. It has been detected in human plasma, following citrus juice intake, at sub-micromolar concentrations, and its main phase II conjugated metabolites (Naringenin-7-O-glucuronide and narigenin-4'-O-glucuronide) have been identified in urine. Recent evidence suggests a potential active anti-inflammatory role of flavonoids on macrophages, cells actively involved in atherogenesis. The aim of this study was to evaluate the effects of naringenin and its phase II metabolites on the expression of specific genes in differently activated macrophages at concentrations coherent with dietary exposure. Results suggest that phase II metabolites, as well as the aglyconic form of naringenin, were able to perturb macrophage gene expression in directions that are not always consistent with anti-inflammatory effects. Moreover, the effects of metabolites were not always consistent with each other and with those of their aglycone, underlining the paramount importance of testing physiological forms of phytochemicals within in vitro experimental models. In vivo studies are needed to further explore these observations and investigate their practical consequences.
Bioavailability and metabolism of orange juice flavanones in humans: impact of a full-fat yogurt.[Pubmed:19007165]
J Agric Food Chem. 2008 Dec 10;56(23):11157-64.
The bioavailability of dietary phytochemicals may be influenced by the food matrix in which they are consumed. In this study the impact of a full-fat yogurt on the bioavailability and metabolism of orange juice flavanones was investigated. Human plasma and urine were collected over a 24 h period after the consumption of 250 mL of orange juice containing a total of 168 micromol of hesperetin-7-O-rutinoside and 12 micromol of naringenin-7-O-rutinoside, with and without 150 mL of full-fat yogurt. The juice also contained 1 g of paracetamol and 5 g of lactulose. HPLC-MS(2) analysis revealed the accumulation of hesperetin-7-O-glucuronide, and an unassigned hesperetin-O-glucuronide metabolite in plasma reached a peak concentration (C(max)) of 924 +/- 224 nmol/L, 4.4 +/- 0.5 h (T(max)) after orange juice ingestion. The T(max) is indicative of absorption in the colon. When the juice was consumed with yogurt, neither the C(max) at 661 +/- 170 nmol/L nor the T(max) at 5.1 +/- 0.4 h were significantly different from those obtained with juice alone. The two hesperetin glucuronides were also excreted in urine along with a third hesperetin-O-glucuronide, two hesperetin-O-glucuronide-O-sulfates, a hesperetin-O-diglucuronide, a naringenin-O-diglucuronide, and, tentatively identified, Naringenin-7-O-glucuronide and naringenin-4'-O-glucuronide. This indicates the occurrence of substantial, postabsorption, phase II metabolism prior to urinary excretion. The quantity of flavanone metabolites excreted 0-5 h after orange juice ingestion was significantly reduced by yogurt, but over the full 0-24 h urine collection period, the amounts excreted, corresponding to ca. 7.0% of intake, were not affected by the addition of yogurt to the drink. Nor did yogurt have a significant effect on gastric emptying, as determined by plasma paracetamol levels, or on the mouth to cecum transit time of the head of the meal, assessed by measurement of lactulose-derived breath hydrogen. There is also a discussion of the merits of studies of the absorption and metabolism of flavanones based on direct analysis of metabolites by HPLC-MS and the more traditional indirect approach where samples are treated with a mollusc glucuronidase/sulfatase preparation prior to HPLC analysis of the released aglycones.
Identification of isomeric flavonoid glucuronides in urine and plasma by metal complexation and LC-ESI-MS/MS.[Pubmed:16810646]
J Mass Spectrom. 2006 Jul;41(7):911-20.
Noncovalent complexes were used for structural determination and isomer differentiation of flavonoid glucuronides. Several flavonoid glucuronides including naringenin-7-O-glucuronide, synthesized here for the first time, were used as test compounds. Electrospray ionization quadrupole ion trap mass spectrometry with collision-induced dissociation (CID) was used to analyze complexes of the form [Co(II) (L-H) (Aux)]+ and [Co(II) (L-H) (Aux)2]+, in which L is the flavonoid glucuronide and Aux is a phenanthroline-based ligand. These complexes yielded characteristic fragmentation patterns that facilitated assignment of the substitution position of the glucuronides. The methods were adapted to liquid chromatography/tandem mass spectrometry (LC-MS/MS) with postcolumn cobalt complexation and were tested on extracts from biological fluids. The metabolites Naringenin-7-O-glucuronide and naringenin-4'-O-glucuronide were detected in human urine following the consumption of grapefruit juice. Isomeric quercetin glucuronides were identified and differentiated after spiking rat plasma at the 1 microM level, proving that the new methods are effective at biologically relevant concentrations.


